|
|
| (24 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| | {{Carnegie No.20 Header}} |
|
| |
|
| | ==Growth and Alteration of Form of the Cartilaginous Canals== |
|
| |
|
| GROWTH AND ALTERATION OF FORM OF THE CARTILAGINOUS CANALS.
| | In embryos 30 mm. long the cartilaginous labyrinth has attained approximately the adult form. Its subsequent development is primarily an increase in size to accommodate the growing membranous labyrinth. If a cast of the superior cartilaginous canal of an 80 mm. embryo by compared with a similar cast of the same canal in a 30 mm. embryo, it will be seen that the general form of the canal in the older specimen is much the same as in the younger specimen But its diameter and length have both increased, the diameter being nearly doubled and the length trebled; furthermore, its linear curvature corresponds to an arc with a considerably longer radius. In reality therefore, the developing cartilaginous labyrinth is continually undergoing changes, both in size and form. The histological evidence of these changes constitutes one of the most interesting features in the development of this region, and although it does not directly concern the development of the contained perioticular spaces, yet it may not be out of place to point out some of the elements of this process as they are seen in our material. In fact, the cartilaginous capsule of the ear is an especially favorable place for studying the general question of growth of cartilage, for two reasons: (1) there are, on account of the intricacy of form of the labyrinth, many kinds of cartilaginous changes found there that are necessary to accommodate its growth, including the deposit of new tissue and the removal of old tissues ; (2) the topography is so well marked by known landmarks that all of these changes as well as the location and direction of growth can be easily followed. |
|
| |
|
| In embryos .'JO mm. long the cartilaginous labyrinth has attained approximateh'
| |
| the adult form. Its subsequent development is primarily an increase in size to
| |
| accommodate the growing membranous labyrinth. If a cast of the superior cartilaginous canal of an 80 mm. embryo b(; compared with a similar cast of the same
| |
| canal in a .30 mm. embryo, it will be seen that the general form of the canal in the
| |
| older specimen is much the same as in the younger specimen But its diameter
| |
| tuid length ha\e both increased, the diameter being nearly doubled and the length
| |
| trebled; furthermore, its linear curvature corresi)onds to an arc with a considerably
| |
| longer radius. In realitj', therefore, the developing cartilaginous labyrinth is continually undergoing changes, both in size and ff)rm. The histological evidence of
| |
| these changes constitutes one of the most interesting features in the development of this region, and although it does not directly concern the development of the contained perioticular spaces, yet it may not be out of place to point out some of the
| |
| elements of this process as they are seen in our material. In fact, the cartilaginous
| |
| capsule of the ear is an especially favorable place for studying the general question
| |
| of growth of cartilage, for two reasons: (1) there are, on account of the intricacy of
| |
| form of the labyrinth, many kinds of cartilaginous changes found there that are
| |
| necessary to accommodate its growth, including the deposit of new tissue and the
| |
| removal of old tissues ; (2) the topography is so well marked bj^ known landmarks
| |
| that all of these changes as well as the location and direction of growth can be
| |
| easily followed.
| |
|
| |
|
| Growth of cartilage is usuallj^ considered to be of two kinds, which are distinguished from each other by being either interstitial or perichondria!. Interstitial growth is described as consisting of an increase in the amount of hyaline | | Growth of cartilage is usually considered to be of two kinds, which are distinguished from each other by being either interstitial or perichondria!. Interstitial growth is described as consisting of an increase in the amount of hyaline matrix and the growth and proliferation of the encapsulated cartilage cells. The new cells form new capsules to a certain extent, but a point is finally reached beyond which the newly prohferated cells continue to occupy their parent capsule. From this variety of growth there results a uniform intumescence of the tissue without producing any marked change in its form. This manner of growth forms a large element in the increase in size of some parts of the capsule of the ear. In those parts, however, where a change in form is involved the growth is more like that described under perichondrial growth and consists of a new deposit of cartilage along the borders of the older cartilage, the consituent cells passing through a precartilage stage. In the otic capsule this latter type of growth is actively going on even before a definite perichondrium is established. The deposit of new cartilage along the margin of older cartilage and the removal of old cartilage by dedifferentiation are indeed the main factors in the process through which the form of the ear-capsule is modeled. |
| matrix and the growth and proliferation of the encapsulated cartilage cells. The | |
| new cells form new capsules to a certain extent, but a point is finall}' reached beyond | |
| which the newly prohferated cells continue to occupy their parent capsule . From | |
| this variety of growth there results a uniform intumescence of the tissue without | |
| producing any marked change in its form. This manner of growth forms a large | |
| element in the increase in size of some parts of the capsule of the ear. In those | |
| parts, however, where a change in form is involved the growth is more Uke that | |
| described under perichondrial growth and consists of a new deposit of cartilage | |
| along the borders of the older cartilage, the consituent cells passing through a precartilage stage. In the otic capsule this latter type of growth is actively going on | |
| even before a definite perichondrium is established. The deposit of new cartilage | |
| along the margin of older cartilage and the removal of old cartilage bj' dedifferentiation are indeed the main factors in the process through which the form of the | |
| ear-capsule is modeled. | |
|
| |
|
| The excavation of established cartilage can be studied by comparing sections
| |
| through the semicircular canals at difTerent stages, such as appear in figures 11, 12,
| |
| 14, and 15. These are all sections through the same canal (lateral), taken in about
| |
| the same position, and are enlarged the same number of diameters. It is, of course,
| |
| possible that they were shrunken in different degrees in the process of embedding;
| |
| this discrepancy, however, is probably not enough to interfere with their showing
| |
| the approximate increase in size of the cartilaginous canal at the respective ages.
| |
| A crude measurement of the perimeters of the canals as seen in the original photographs (100 diameters) yields the following circumferences: 30-mm. embryo, 115
| |
| mm. circumference; 37-mm. embryo, 132 nam. circumference; 43-mm. embryo, 152
| |
| mm. circumference; 50-mm. embryo, 192 mm. circumference. It is evident that we
| |
| are dealing with an enlarging space and that a study of its receding edge must give
| |
| the histological picture of the replacement of true cartilage by other tissue, either
| |
| by dedifferentiation or by direct metaplasia.
| |
|
| |
|
| If, with this process in mind, one makes an examination of the specimen shown
| | The excavation of established cartilage can be studied by comparing sections through the semicircular canals at different stages, such as appear in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 11, 12]], [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_2|14, and 15]]. These are all sections through the same canal (lateral), taken in about the same position, and are enlarged the same number of diameters. It is, of course, possible that they were shrunken in different degrees in the process of embedding; this discrepancy, however, is probably not enough to interfere with their showing the approximate increase in size of the cartilaginous canal at the respective ages. A crude measurement of the perimeters of the canals as seen in the original photographs (100 diameters) yields the following circumferences: 30-mm. embryo, 115 mm. circumference; 37-mm. embryo, 132 mm. circumference; 43-mm. embryo, 152 mm. circumference; 50-mm. embryo, 192 mm. circumference. It is evident that we are dealing with an enlarging space and that a study of its receding edge must give the histological picture of the replacement of true cartilage by other tissue, either by dedifferentiation or by direct metaplasia. |
| in figure 11 (Carnegie Collection, No. 86) it will be seen under higher magnification | |
| that a rather definite border can be made out separating the general mass of true cartilage from the inner zone of temporarj'^ precartilage surrounding the semicircular ducts. The true cartilage has develojjed a consideral)le amount of matrix | |
| separating the encapsulated nuclei or cartilage cells. The margins of the capsules
| |
| stand out as sharp refractive lines. The matrix lying between the capsules is
| |
| slight I}- opaque and is beginning to take a differential stain. A narrow intermediate or transition zone separates the true cartilage from the precartilage; this zone
| |
| is characterized by the presence of flattened and i)artially collapsed capsules between | |
| which there is very little or no matrix. The refractive margins of these overlai)iMng, incomplete capsules give the appearance of wavy lines that run parallel
| |
| with the margin of the canal. The same appearance is not seen in other regions of
| |
| the otic capsule in younger stages, where precartilage is differentiating into cartilage.
| |
|
| |
|
| In the process of cartilage differentiation in most parts of the otic capsule
| |
| there is considerable intercapsular material at the time the margins of the capsules become conspicuous. The capsules
| |
| are separated by the matrLx-forming syncytium. Thus, there are not the conspicuous wavy lines due to overlajJijing
| |
| capsules, such as characterize the intermediate zone. The transition between
| |
| this zone and the true cartilage on one
| |
| hand and the temporary precartilage on
| |
| the other is quite abrupt in both instances. On entering the zone of j)recartilage there is found between the
| |
| nuclei, instead of the wavy refractive
| |
| capsular lines, a framework having more
| |
| the character of a granular syncj'tium,
| |
| with only here and there the suggestion
| |
| of a Ix'gimiing capsule. This, it will be
| |
| remembered, is a condition the true cartilage exhibited in its earlier i)eriod. It
| |
| is the intermediate zone to which we
| |
| should address our especial attention,
| |
| and it is this zone that moves outward
| |
| as the cartilaginous canal widens.
| |
|
| |
|
| Text-figure 1 .shows a section
| | If, with this process in mind, one makes an examination of the specimen shown in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 11]] (Carnegie Collection, No. 86) it will be seen under higher magnification that a rather definite border can be made out separating the general mass of true cartilage from the inner zone of temporary precartilage surrounding the semicircular ducts. The true cartilage has develojjed a consideral)le amount of matrix separating the encapsulated nuclei or cartilage cells. The margins of the capsules stand out as sharp refractive lines. The matrix lying between the capsules is slightly opaque and is beginning to take a differential stain. A narrow intermediate or transition zone separates the true cartilage from the precartilage; this zone is characterized by the presence of flattened and partially collapsed capsules between which there is very little or no matrix. The refractive margins of these overlaying, incomplete capsules give the appearance of wavy lines that run parallel with the margin of the canal. The same appearance is not seen in other regions of the otic capsule in younger stages, where precartilage is differentiating into cartilage. |
| througli the.sc zones in a fetus of about
| |
| the same age as the one just described. | |
| This section is taken through the lateral
| |
| length (Carnegie Collection, No. 145).
| |
|
| |
|
|
| |
|
| | In the process of cartilage differentiation in most parts of the otic capsule there is considerable intercapsular material at the time the margins of the capsules become conspicuous. The capsules are separated by the matrix-forming syncytium. Thus, there are not the conspicuous wavy lines due to overlaying capsules, such as characterize the intermediate zone. The transition between this zone and the true cartilage on one hand and the temporary precartilage on the other is quite abrupt in both instances. On entering the zone of precartilage there is found between the nuclei, instead of the wavy refractive capsular lines, a framework having more the character of a granular syncytium, with only here and there the suggestion of a Ix'giming capsule. This, it will be remembered, is a condition the true cartilage exhibited in its earlier period. It is the intermediate zone to which we should address our especial attention, and it is this zone that moves outward as the cartilaginous canal widens. |
|
| |
|
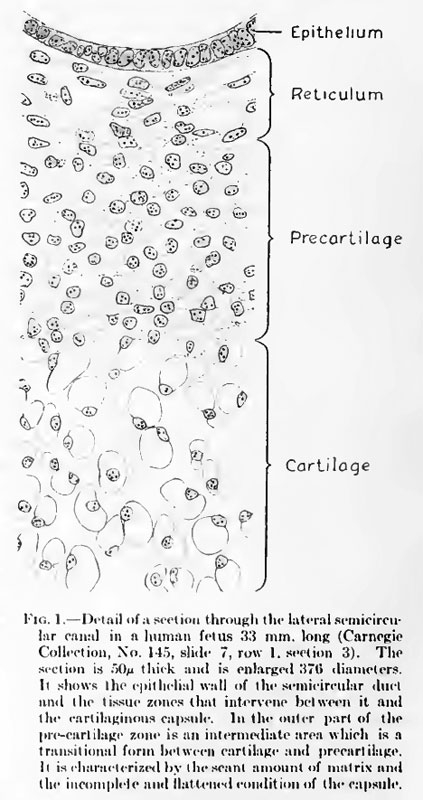
| | {| |
| | | <div id="Fig01"></div> |
| | [[File:Streeter001.jpg]] |
| | | '''Fig. 1. Detail of a section through tlie lateral semicircular canal in a human fetus 33 mm long''' |
| | :(Carnegie Collection, No. 145, slide 7, row 1, section 3). The section is 50 microns thick and is enlarged 370 diameters. It shows the epithelial wall of the semicircular duct and the tissue zones that intervene between it and the cartilaginous capsule. In the outer part of the pre-cartilage zone is an intermediate area which is a transitional form between cartilage and precartilage. It is characterized by the scant amount of matrix and the incomplete and flattened condition of the capsule. |
| | |} |
|
| |
|
| Fig. 1. — Detail of a section through tlie lateral .wmicircular oaiial in a human fetus 33 mm. long (Carnegie
| | Text-figure 1 shows a section through these zones in a fetus of about the same age as the one just described. This section is taken through the lateral canal of a fetus 33 mm. crown-rump length (Carnegie Collection, No. 145). |
| Collection, No. 14.'j, slide 7, row 1, section 3). The | |
| section is .")(V thick and is enlarge*! 37() diameters.
| |
| It shows the epithelial wall of the seniieireular duct
| |
| and the tissue zones that intervene between it and
| |
| the cartilaginous capsule. In the outer part of llie
| |
| J (re-cartilage zone is an intermediate area which is a
| |
| transitional form between cartilage an<l precartilage.
| |
| It is characterizetl by the scant .amount of matrix and
| |
| the incomplete and flattened c(in<lilion of the cap.sule.
| |
|
| |
|
| canal of a fetus 33 mm. crown-rump | | The intermediate zone stands out conspicuously at the junction of the cartilage with the precartilage. Its wavy refractive lines are so compact that under low powers the whole zone appears as a dark rim outlining the cartilaginous margin of the canal. The compactness of these lines varies in different embryos of about the same age and even varies in the two borders of a given canal. |
|
| |
|
| The intermediate zone stands out conspicuously at the junction of the cartilage with the precartilage. Its wavy refractive lines are so compact that under low powers the whole zone appears as a dark
| | This latter condition can be seen in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_2|figure 14]], which represents the lateral semicircular canal of a fetus 43 mm. crown-rump length (Carnegie Collection, No. 886). It will be noted that the peripheral two-thirds of the intermediate zone (toward the right hand) forms a dark, heavy margin between the true cartilage and the encircled precartilage, whereas the central one-third (toward the left hand) is wider and much less distinct. It can also be seen that the place at which this intermediate zone is well marked corresponds to the direction of the excavation necessary to allow for the growth of the canal and to make room for the elongating semicircular ducts of the contained membranous labyrinth. In this case the expansion must be toward the periphery of the cartilaginous capsule, i.e., toward the right side of the photograph. From studying various fetuses it seems to be true that where excavation of cartilage is actively going on at such a place there is found a prominent intermediate zone along the inner margin of the cartilage. Sometimes the line is uniform around the entire rim, but usually it is more marked on one side of the canal than on the other, and in such cases it is always toward the direction of the excavation of the cartilage, as can be judged from the topography of the labyrinth. |
| rim outlining the cartilaginous margin of the canal. The compactness of these
| |
| lines varies in different embryos of about the same age and even varies in the two
| |
| borders of a given canal.
| |
|
| |
|
| This latter condition can be seen in figure 14, which represents the lateral semicircular canal of a fetus 43 mm. crown-rump length (Carnegie Collection, No. 886).
| | If an older specimen is examined, such as the one represented in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_2|figure 15]], the character and relative position of the cartilage and precartilage are found to be the same as in the 30-mm. stage just described. They have, however, undergone an alteration to allow for the enlargement of the cartilaginous canal. [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_2|Figure 15]] shows the lateral canal in a human fetus about 50 mm. crown-rump length (Carnegie Collection, No. 95). The fetus is catalogued as being 46 mm. long, but this is apparently the slide-measurement. In its development it corresponds to fetuses 50 mm. long, formalin measurement, and this measurement is used so that it will accord with the other fetuses. Since figures 11 and 15 represent sections through the same canal taken at about the same place and under the same enlargement, one can superimpose them, one upon the other, and thus determine the change that has occurred between the two stages. If this is done it will be seen that the area that was precartilage in the 30-mm. stage is replaced by reticulum in the 50-mm. stage. There is just as much or more precartilage in the latter, but it ha.s moved outward into the area that was previously true cartilage. In other words, the enlargement of the cartilaginous canal has been obtained by a process of excavation based on the dedifferentiation of true cartilage into precartilage and the latter in turn into reticulum. This is shown under higher magnifications in text-figures 2 and 3, which show sections of these same canals under the same enlargement and placed side by side for the purpose of better comparison. It can be seen in these two figures how the cartilage of 30-mm. stage becomes dedifferentiated into the precartilage of the 50-mm. stage and the border along which this process is in active operation forms the intermediate zone, which is characterized by its wavy, refractile lines. The precartilage in turn is gradually dedifferentiated into the periotic reticulum. In this way the margin of the true cartilage gradually recedes from the epithelial duct, and the last of the precartilage is eventually dedifferentiated into a reticulum. Along with the process of excavation of cartilage there must go the laying-down of new cartilage. For instance, as the lateral cartilalaginous canal enlarges it also moves laterally, so that the distance between it and the cartilaginous vestibule increases, producing relatively a lateral migration of the si^ace as a whole. Such a migration involves the excavation of the established cartilage on its lateral margin and the formation of new cartilage on its median margin. On its laveral margin true cartilage is being dedifferentiated into precartilage and on its median margin precartilage is differentiating into cartilage. It is this phenomenon that determines the conditions shown in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_2|figure 14]]. On the right can be seen the prominent intermediate zone, indicating an active excavation of cartilage, and on the left the line of transition between cartilage and precartilage presents the same picture as that seen in the stage of differentiation of the latter into the former. One is forced to conclude that the cartilaginous tissue of the otic capsule is callable of differentiation and dedifferentiation in its earlier stages, at least up to the time of the completion of the encapsulation of the cartilage cells. This progressive and retrogressive adaptability of the cartilaginous tissue makes possible the changes that are necessary in the growth and alteration in form of the labyrinth. |
| It will be noted that the peripheral two-thirds of the intermediate zone (toward the
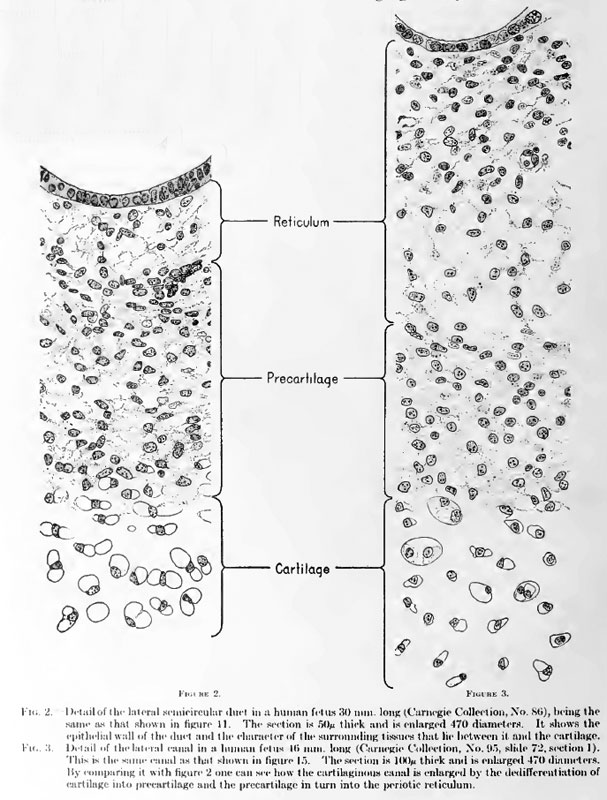
| | {| |
| right hand) forms a dark, heavy margin between the true cartilage and the encircled
| | | <div id="Fig02"></div> |
| precartilage, whereas the central one-third (toward the left hand) is wider and much | | <div id="Fig03"></div> |
| less distinct. It can also be seen that the place at which this intermediate zone is
| | [[File:Streeter002-3.jpg]] |
| well marked corresponds to the direction of the excavation necessary to allow for the
| |
| growth of the canal and to make room for the elongating semicircular ducts of the
| |
| contained membranous labyrinth. In this case the expansion must be toward the
| |
| periphery of the cartilaginous capsule, i.e., toward the right side of the photograph.
| |
| From studying various fetuses it seems to be true that where excavation of cartilage
| |
| is actively going on at such a place there is found a prominent intermediate zone along | |
| the inner margin of the cartilage. Sometimes the line is uniform around the entire | |
| rim, but usually it is more marked on one side of the canal than on the other, and
| |
| in such cases it is always toward the direction of the excavation of the cartilage, | |
| as can be judged from the topography of the labyrinth.
| |
|
| |
|
| If an older specimen is examined, such as the one represented in figure 15, the
| | | '''Fig. 2. Detail of the lateral semicircular duct in a human fetus 30 mm. long''' |
| character and relative position of the cartilage and precartilage are found to be the
| | :(Carnegie Collection, No. 86), being the same as that shown in figure 11. The section is 50 micron thick and is enlarged 470 diameters. It shows the epithelial wall of the duct and the character of the surrounding tissues that lie between it and the cartilage. |
| same as in the 30-mm. stage just described. They have, however, undergone an
| |
| alteration to allow for the enlargement of the cartilaginous canal. Figure 15 shows
| |
| the lateral canal in a human fetus about 50 mm. crown-rump length (Carnegie | |
| Collection, No. 95). The fetus is catalogued as being 46 mm. long, but this is | |
| apparently the slide-measurement. In its development it corresponds to fetuses
| |
| 50 mm. long, formalin measurement, and this measurement is used so that it will
| |
| accord with the other fetuses. Since figures 11 and 15 represent sections through
| |
| the same canal taken at about the same place and under the same enlargement, one | |
| can superimpose them, one upon the other, and thus determine the change that
| |
| has occurred between the two stages. If this is done it will be seen that the area
| |
| that was precartilage in the 30-mm. stage is replaced by reticulum in the 50-mm.
| |
| stage. There is just as much or more precartilage in the latter, but it ha.s moved outward into the area that was previously true cartilage. In other words, the enlargement of the cartilaginous canal has been obtained by a process of excavation based on
| |
| the dedifferentiation of true cartilage into precartilage and the latter in turn into
| |
| reticulum. This is shown under higher magnifications in text-figures 2 and 3,
| |
| which show sections of these same canals under the same enlargement and placed
| |
| side by side for the purpose of better comparison. It can be .seen in these two
| |
| figures how the cartilage of 30-mm. stage becomes dedifferentiated into the precartilage of the 50-mm. stage and the border along which this process is in active
| |
| operation forms the intermediate zone, which is characterized by its wavy, refractile lines. The precartilage in tuin it^ gradualh' dedifferentiated into the periotic reticukim. In this way the margin of the true cartilage graduall.y recedes from tlie
| |
| epithelial duct, and the last of the precartilage
| |
| is eventually dedifferentiated into a reticulum.
| |
| Along with the process of excavation of cartilage there must go the laying-down of new
| |
| cartilage. For instance, as the lateral cartilalaginous canal enlarges it also moves laterally,
| |
| so that the distance between it and the cartilaginous vestibule increases, producing relatively a lateral migration of the si^ace
| |
| as a whole. Such a migration involves the excavation of the established cartilage
| |
| on its lateral margin and the formation of new cartilage on its median margin. On
| |
| its laveral margin true cartilage is being dedifferentiated into precartilage and on
| |
| its median margin precartilage is differentiating into cartilage. It is this phenomenon that determines the conditions shown in figure 14. On the right can be
| |
| seen the prominent intermediate zone, indicating an active excavation of cartilage,
| |
| and on the left the line of transition between cartilage and precartilage presents
| |
| the same picture as that seen in the stage of differentiation of the latter into the
| |
| former. One is forced to conclude that the cartilaginous tissue of the otic capsule
| |
| is callable of differentiation and dedifferentiation in its earlier stages, at least up to
| |
| the time of the completion of the encapsulation of the cartilage cells. This progressive and retrogressive adaptability of the cartilaginous tissue makes possible the
| |
| changes that are necessary in the growth and alteration in form of the labyrinth.
| |
|
| |
|
|
| |
|
| FlGURK 2.
| |
|
| |
|
| Flo. '2.--Detailof the lateral semicircular duct in a human fetus 30 mm. long (Carnegie Collection, No. 86), being the
| |
| same as that shown in figure 11. The section is .'50^ thick and is enlarged 470 diameters. It shows the
| |
| epithelial wall of the duct and the <'hiir;icter of the surrouiiiliii); tissues thai lie between it and the cartilage.
| |
|
| |
|
| FlO. 3.— Detail of the lateral canal iti a human felus Iti niiii. lorifl (Carnegie ( 'oUeil ion, No. !).">, slide 72, section 1).
| |
| This i.s the same canal as that shown in tiguie I."). The .section is l(K)/u thick and is enlarged 470 diameters.
| |
| By comparing it with figure 2 one can see how the cartilaginous canal is enlarged by the tledilTerciitiation of
| |
| cartilage into precartilage and the precartilage in turn into the periotic reticulum.
| |
|
| |
|
| | '''Fig. 3. Detail of the lateral canal in a human fetus 16 mm. long''' |
| | :(Carnegie Collection, No. 95, slide 72, section 1). This is the same canal as that shown in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_2|figure 15]]. The section is 100 micron thick and is enlarged 470 diameters. By comparing it with figure 2 one can see how the cartilaginous canal is enlarged by the dedifferentiation of cartilage into precartilage and the precartilage in turn into the periotic reticulum. |
| | |} |
|
| |
|
| DEVELOPMENT OF PERIOTIC RETICULAR CONNECTIVE TISSUE.
| | ==Development of Periotic Reticular Connective Tissue== |
|
| |
|
| The formation of the connective-tissue reticulum surrounding the semicircular | | The formation of the connective-tissue reticulum surrounding the semicircular ducts is first indicated by a cluster of darkly stained nuclei that lie along the central edge of the ducts in embryos soon after the ducts are formed and before the differentiation of the cartilage is completed. In figure 9 such a cluster is seen just under the posterior duct in the upper part of the photograph. In [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 10]], which shows the lateral semicircular duct of an embryo 27 mm. long (Carnegie Collection, No. 756a), a similar cluster of nuclei can be seen just under the duct, in reaUty just median to it. These foci mark the points at which the formation of the reticulum begins. It is not, however, until we come to embryos about 30 nmi. long that we find a definite reticulum. At that time, as is shown in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 11]], a narrow lighter area can be made out, situated between the epithelial wall of the duct and the temporary precartilage. It is the development of this area at the expense of the temporary precartilage that results in the reticulum in which the periotic spaces are subsequently formed. This area consists of a mesenchymal syncytium containing irregularly shaped clear tissue spaces and is characterized by the presence of numerous blood-vessels and connecting capillaries. The larger vessels are found resting against the inner margin of the temporary precartilage. They sometimes indent it, but never penetrate it to any extent. Such vessels can be seen in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 11 and 12]]. The presence of these blood-vessels is coincident with the appearance of the reticular tissue. |
| ducts is first indicated by a cluster of darkly stained nuclei that lie along the central | |
| edge of the ducts in embryos soon a^ter the ducts are formed and before the differentiation of the cartilage is completed. In figure 9 such a cluster is seen just under | |
| the posterior duct in the upper part of the photograph. In figure 10, which shows | |
| the lateral semicircular duct of an embryo 27 mm. long (Carnegie Collection, No. | |
| 756a), a similar cluster of nuclei can be seen just under the duct, in reaUty just | |
| median to it. These foci mark the points at which the formation of the reticulum | |
| begins. It is not, however, until we come to embryos about 30 nmi. long that we | |
| find a definite reticulum. At that time, as is shown in figure 11, a narrow fighter | |
| area can be made out, situated between the epithelial wall of the duct and the | |
| temporary precartilage. It is the development of this area at the expense of the | |
| temporary precartilage that results in the reticulum in which the periotic spaces are | |
| subsequently formed. This area consists of a mesenchymal syncytium containing | |
| irregularly shaped clear tissue spaces and is characterized by the presence of numerous blood-vessels and connecting capillaries. The larger vessels are found resting | |
| against the inner margin of the temporary precartilage. They sometimes indent it, | |
| but never penetrate it to any extent. Such vessels can be seen in figures 11 and 12. | |
| The presence of these blood-vessels is coincident with the appearance of the reticular tissue. | |
|
| |
|
| In describing younger stages the statement has been made that the temporary
| |
| precartilage abuts directly against the epithelial wall of the semicircular duct.
| |
| This statement is based only on the gross appearance. On careful scrutiny of the
| |
| tissue that immediately surrounds the ducts in embryos between 14 mm. and 20
| |
| mm. long a few mesenchymal cells can be found which possibly do not belong to
| |
| the tenijjorary precartilage. These cells may very well represent some of the
| |
| indifferent mesenchyme, and possibly also some angioblasts. It is conceivable
| |
| that these surround the otic vesicle in its earUest stages and are inclosed along with the Otic vesicle by the condensed tissue of the otic capsule, where they remain in
| |
| contact with the epithelial labyrinth in a resting condition until the embryo approaches 20 mm. in length. They then show activity and by the time the embryo
| |
| is 30 mm. long we find them converted into a vascularized reticulum which forms a
| |
| definite area surrounding each semicircular duct and completely separating it from
| |
| the receding precartilage. The area of reticulum advances as the precartilage
| |
| becomes hollowed out. This can be seen by comparing figur(>s 11, 12, 14, 15, and
| |
| 16, all of which are reproduced on the same scale of enlargement.
| |
|
| |
|
| From the histological api)earance one could maintain that the reticuhmi is
| | In describing younger stages the statement has been made that the temporary precartilage abuts directly against the epithelial wall of the semicircular duct. This statement is based only on the gross appearance. On careful scrutiny of the tissue that immediately surrounds the ducts in embryos between 14 mm. and 20 mm. long a few mesenchymal cells can be found which possibly do not belong to the temporary precartilage. These cells may very well represent some of the indifferent mesenchyme, and possibly also some angioblasts. It is conceivable that these surround the otic vesicle in its earUest stages and are inclosed along with the Otic vesicle by the condensed tissue of the otic capsule, where they remain in contact with the epithelial labyrinth in a resting condition until the embryo approaches 20 mm. in length. They then show activity and by the time the embryo is 30 mm. long we find them converted into a vascularized reticulum which forms a definite area surrounding each semicircular duct and completely separating it from the receding precartilage. The area of reticulum advances as the precartilage becomes hollowed out. This can be seen by comparing [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 11, 12, 14, 15, and |
| derived from a few predestined mesenchymatous cells which, after a latent period,
| | 16]], all of which are reproduced on the same scale of enlargement. |
| undergo proliferation and occupy the space that is vacated by the receding precartilage in the manner described above, the growth of the reticulum perhaps being
| |
| the cause of the recession of the cartilage. But one could eciually well maintain | |
| that the reticulum is derived entirely from the precartilage; that it is not a predetermined tissue, but simply precartilage that has undergone dedifferentiation.
| |
| It is entirely possible that the isolated cells included with the epithelial labyrinth | |
| are angioblasts only, everything else being indifferent mesenchyme. In the early
| |
| stages, where only a few cells are concerned, this matter can not be determined,
| |
| the histological difference between early precartilage and other embryonic cells not | |
| being sufficiently great for their certain recognition. In the later stages, however,
| |
| it is quite evident that precartilage tissue is actually converted into a reticulum;
| |
| that the replacement of the temporary precartilage by a reticular connective tissue is
| |
| accompUshed by a process of dedifferentiation, or direct metaplasia, just as we have
| |
| previously seen in the case of the dedifferentiation of cartilage into precartilage.
| |
|
| |
|
| In this connection it is instructive to compare again figures 11 and 15, and also
| |
| fig\ires 2 and 3, which are details of the same under higher magnification. They
| |
| .show under the same enlargement a section through the lateral canal made in about
| |
| the same position and cut at the same thickness. It will be noticed that the space
| |
| occupied by precartilage in the younger stage is entirely filled in by reticulum in
| |
| the older stage. There is in the older stage, however, more precartilage than before,
| |
| but it now occupies a more peripheral j^osition. With the change in the position
| |
| of the precartilage area there is a corresponding enlargement of the lumen of the
| |
| true cartilage, i. e., the cartilaginous canal. It is clear that we are dealing here
| |
| with a dedifferentiation of true cartilage into precartilage on the one hand and a
| |
| dedifferentiation of precartilage into reticulum on the other. These factors, as we
| |
| already have seen, are of great imjiortance in the alteration in form and size of the
| |
| cartilaginous canals.
| |
|
| |
|
| In younger stages, as in figure 10, the epithelial semicircular duct lies near the
| | From the histological api)earance one could maintain that the reticulum is derived from a few predestined mesenchymatous cells which, after a latent period, undergo proliferation and occupy the space that is vacated by the receding precartilage in the manner described above, the growth of the reticulum perhaps being the cause of the recession of the cartilage. But one could eciually well maintain that the reticulum is derived entirely from the precartilage; that it is not a predetermined tissue, but simply precartilage that has undergone dedifferentiation. It is entirely possible that the isolated cells included with the epithelial labyrinth are angioblasts only, everything else being indifferent mesenchyme. In the early stages, where only a few cells are concerned, this matter can not be determined, the histological difference between early precartilage and other embryonic cells not being sufficiently great for their certain recognition. In the later stages, however, it is quite evident that precartilage tissue is actually converted into a reticulum; that the replacement of the temporary precartilage by a reticular connective tissue is accompUshed by a process of dedifferentiation, or direct metaplasia, just as we have previously seen in the case of the dedifferentiation of cartilage into precartilage. |
| center of the area of temjiorary pre(;artilage. ^^'hen the reticulum de\elops it
| |
| makes its first appearance, and its growth continues more marked along the concave
| |
| side of the duct than on the convex side— that is, on the side toward the utricle
| |
| rather than toward the ix'rii)hery of the capsule. On this account the epithelial
| |
| duct lo.ses its central position and gradually comes to lie along the peripheral border
| |
| of the cartilaginous canal, where it eventuallj' becomes attached to the periosteum. | |
|
| |
|
| This eccentric position gives the canal the largest arc that is possible in the space in
| |
| which it lies. It marks the point of thrust of the elongating duct against the cartilaginous chamber that confines it and it is in this direction that the cartilage must be
| |
| excavated to make room for the further growth of the duct.
| |
|
| |
|
| The spread of the reticulum into the surrounding precartilage is rather slow at
| | In this connection it is instructive to compare again [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 11 and 15]], and also figures 2 and 3, which are details of the same under higher magnification. They show under the same enlargement a section through the lateral canal made in about the same position and cut at the same thickness. It will be noticed that the space occupied by precartilage in the younger stage is entirely filled in by reticulum in the older stage. There is in the older stage, however, more precartilage than before, but it now occupies a more peripheral position. With the change in the position of the precartilage area there is a corresponding enlargement of the lumen of the true cartilage, i. e., the cartilaginous canal. It is clear that we are dealing here with a dedifferentiation of true cartilage into precartilage on the one hand and a dedifferentiation of precartilage into reticulum on the other. These factors, as we already have seen, are of great importance in the alteration in form and size of the cartilaginous canals. |
| first. There is very Uttle advance made in fetuses between 30 mm. and 43 mm.
| |
| long, as can be seen by comparing figures 11 to 14. In figure 14 the reticulum can
| |
| be recognized as a crescentic-shaped area on the central side (toward the left) and
| |
| partially surrounding the epithelial duct. In the figure it is about 0.8 cm. wide at
| |
| its widest point. The surrounding precartilage is also of about the same \\ddth,
| |
| but it is uniformly wide around the whole circumference of the cartilaginous canal. | |
| In fetuses about 50 mm. long the dedifferentiation of precartilage into reticulum
| |
| makes more rapid progress. The change is quite abrupt at this time. Figures 14,
| |
| 15, 16, and 17 form a series in which is shown the alteration from a small amount
| |
| of reticulum to an almost complete reticularization of the cartilaginous canal.
| |
| These changes are found in fetuses varying from 43 mm. to 52 mm. long. In comparing these figures one would e.xpect that the membranous duct would be found
| |
| progressive!}^ larger in the series of photographs if they were correctly arranged in
| |
| the order of their age. But it should be remembered that the tissues show different
| |
| degrees of response to the fixing reagents. This is particularly so in respect to the
| |
| epithelial duct; in figures 14 and 17 it is distended, as can be seen by its thin wall,
| |
| while in 15 and 16 it is contracted. The order in which they are arranged corresponds to their relative age, as far as could be determined by the records of the
| |
| fetuses and general appearances of the sections.
| |
|
| |
|
| In figure 15 there is a zone of precartilage, about 0.8 cm. wide in the photograph,
| |
| which in reality is true cartilage that has been dedifferentiated into precartilage.
| |
| The reticulum extends from the inner border of this to the membranous duct. In
| |
| figure 16, which is a section through the posterior canal of a fetus 50 mm. long
| |
| (Carnegie Collection, No. 184), the dedifTerentiation of precartilage into reticulum
| |
| has occurred faster than that of cartilage into precartilage. There is practically
| |
| none of the latter to be seen; the whole of the space between the margin on the
| |
| cartilage and membranous semicircular duct is filled in by reticulum. Along the
| |
| central margin of the duct there are still seen thick clusters of proliferating nuclei
| |
| which are associated in part with the development of the blood-vessels and in part
| |
| with the modification of the reticulum that takes place around the wall of the
| |
| membranous duct.
| |
|
| |
|
| It has been noted that precartilage is free of blood-vessels, whereas the reticulum is vascularized from the very first. Part of the dedifferentiation of precartilage into reticulum consists of the invasion of blood-vessels into the precartilage
| | In younger stages, as in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 10]], the epithelial semicircular duct lies near the center of the area of temjiorary pre(;artilage. When the reticulum develops it makes its first appearance, and its growth continues more marked along the concave side of the duct than on the convex side that is, on the side toward the utricle rather than toward the ix'rii)hery of the capsule. On this account the epithelial duct loses its central position and gradually comes to lie along the peripheral border of the cartilaginous canal, where it eventuallj' becomes attached to the periosteum. |
| region. In the early stages of the reticulum the larger vessels hug closely against
| |
| the precartilage and continue to do so as the latter recedes from the epithelial | |
| duct, as can be seen in figures 11, 12, and 14. Later, with the abrupt dedifferentiation of the remaining precartilage into reticulum, the larger vessels do not -follow | |
| the receding margin of the cartilaginous canal, but form vascular arches that are suspeiulod in the reticulum, as can be seen in figures 15, 1(), and 17, and from these
| |
| a network of small vessels branches toward the membranous duct on the one hand
| |
| and the cartilaginous wall on the other.
| |
|
| |
|
| In figure 17, which is a section through the posterior semicircular canal in a
| |
| fetus 52 mm. crown-rumi> length (Carnegie Cf)llection, No. 96), the reticulum is
| |
| more mature in its appearance than any that have thus far been described. There
| |
| is practically no precartilage to be seen. The reticulum now only lacks the
| |
| membrane-like thickening of its inner and outer margins to render it complete. At
| |
| the inner margin the cells arrange themselves into a fibrous coat that constitutes the
| |
| memlirana proi^ria of the membranous duct. At the outer margin is formed the
| |
| perichondrium, the development of which will now be considered.
| |
|
| |
|
| DEVELOPMENT OF THE PERICHONDRIUM.
| | This eccentric position gives the canal the largest arc that is possible in the space in which it lies. It marks the point of thrust of the elongating duct against the cartilaginous chamber that confines it and it is in this direction that the cartilage must be excavated to make room for the further growth of the duct. |
|
| |
|
| In the description of the development of the periotic reticulum we have seen
| |
| how it begins as a small focus along the central border of the epithelial semicircular duct and spreads at the expense of the temporary precartilage, forming as it
| |
| does so a crescentic-shaped area of reticulum inclosing the duct. We have also
| |
| seen how the im-asion or spread of the reticulum into the surrounding area of precartilage is brought about, at least in the later stages, by a dedifferentiation of the
| |
| latter into the former.
| |
|
| |
|
| Furthermore, along with this latter process, the inner margin of cartilage surrounding the duct is dedifferentiated into precartilage, so that a new area of precartilage becomes established as the old area disappears. The conversion of precartilage into reticulum in the later stages, however, is more rapid than the conversion
| | The spread of the reticulum into the surrounding precartilage is rather slow at first. There is very Uttle advance made in fetuses between 30 mm. and 43 mm. |
| of cartilage into precartilage, and consefjuently there comes a time when the precartilage has nearly all disappeared. In such specimens the reticuhnn extends
| | long, as can be seen by comparing [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 11 to 14]]. In figure 14 the reticulum can be recognized as a crescentic-shaped area on the central side (toward the left) and partially surrounding the epithelial duct. In the figure it is about 0.8 cm. wide at its widest point. The surrounding precartilage is also of about the same width, but it is uniformly wide around the whole circumference of the cartilaginous canal. In fetuses about 50 mm. long the dedifferentiation of precartilage into reticulum makes more rapid progress. The change is quite abrupt at this time. [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|Figures 14, |
| practicall\' from the epithelial duct to the margin of the cartilaginous canal. The
| | 15, 16, and 17]] form a series in which is shown the alteration from a small amount of reticulum to an almost complete reticularization of the cartilaginous canal. These changes are found in fetuses varying from 43 mm. to 52 mm. long. In comparing these figures one would e.xpect that the membranous duct would be found progressively larger in the series of photographs if they were correctly arranged in the order of their age. But it should be remembered that the tissues show different degrees of response to the fixing reagents. This is particularly so in respect to the epithelial duct; in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 14 and 17]] it is distended, as can be seen by its thin wall, while in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|15 and 16]] it is contracted. The order in which they are arranged corresponds to their relative age, as far as could be determined by the records of the fetuses and general appearances of the sections. |
| (|ualifying term "practically" is used because the inner and outer margins of the
| |
| reticulum are modified in a special manner. The inner margin becomes condensed
| |
| into a membrane-like coat of fibrous tissue that constitutes the membrana ])ropria
| |
| of the membranous canal. The outer margin at about this time undergoes changes
| |
| that result in the formation of the jjerichondrium.
| |
|
| |
|
| In di.scussing the lu'richondrium it is important to kcej) in imnd the active
| |
| alterations in the tissue along the margin of the cartilage that accomj^any the
| |
| growth of the labyrinth. It has been seen how the enlargement of the cartilaginous canals and their alterations in form and position is obtained partly by excavation of cartilage and partly by the laying down of new cartilage, the excavation
| |
| being accomplished by its dedifferentiation into ])recartilage and reticulum, and the
| |
| new cartilage being l>uilt up through a i)recartilage stage from the periotic reticular tissue. Throughout the entire period of growth of the cartilaginous canals
| |
| the elements of this continual transformation exist along their margin. The margin
| |
| during this period is in a state of temporarj' eciuilibrium and is cai)able of advancing or receding as the conditions determine.
| |
|
| |
|
| The first and relatively the major part of the hollowing-out of the cartilaginous
| | In [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 15]] there is a zone of precartilage, about 0.8 cm. wide in the photograph, which in reality is true cartilage that has been dedifferentiated into precartilage. The reticulum extends from the inner border of this to the membranous duct. In [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 16]], which is a section through the posterior canal of a fetus 50 mm. long (Carnegie Collection, No. 184), the dedifferentiation of precartilage into reticulum has occurred faster than that of cartilage into precartilage. There is practically none of the latter to be seen; the whole of the space between the margin on the cartilage and membranous semicircular duct is filled in by reticulum. Along the central margin of the duct there are still seen thick clusters of proliferating nuclei which are associated in part with the development of the blood-vessels and in part with the modification of the reticulum that takes place around the wall of the membranous duct. |
| canals is complete before the perichondrium makes its appearance. This is illustrated, for instance, by the fetus of 52 mm. crown-rump length, in figure 17, where
| |
| there is as yet no indication of it shown. In fetuses between 40 and 50 mm. long | |
| the zone of precartilage surrounding the margins of the canals, as seen in figures
| |
| 14 and 15. might be mistaken for perichondrium. This area, however, in fetuses
| |
| sUghtly older is converted almost entirely into reticulum. Kolliker (1879), in the
| |
| second edition of his text-book on embryologj', pictures a transverse section
| |
| through the lateral canal of a rabbit embryo (fig. 457, page 735), in which this | |
| zone of precartilage is labeled as periosteum of the future bone.
| |
|
| |
|
| The real perichondrium does not make its appearance until the fetus reaches a
| |
| a length of about 70 mm. A specimen of this age is represented in te.xt-figure 4,
| |
| which shows a segment of the posterior semicircular canal in a fetus 73 mm. crownrump length (Carnegie Collection, No. 1373). On examination of this specimen it is
| |
| found that there is a distinct condensation of the reticulum along its inner margin, so
| |
| that it forms a membrana propria for the epithehal duct with which it is in contact.
| |
| This area has largely lost its reticular character and now resembles embryonic
| |
| fibrous connective tissue. Along the outer margin of the reticulum a similar condensation of its trabeculse has taken place, forming a thin fibrous lamina or membrane near the margin of the cartilage. This is the perichondrium in its early
| |
| form. It does not abut directly against the cartilage, but is separated from it by
| |
| a thin layer of transition tissue that is in process of dedifferentiation from precartilage into reticulum.
| |
|
| |
|
| Passing inward from the cartilage, the transitions are rapid from cartilage to
| | It has been noted that precartilage is free of blood-vessels, whereas the reticulum is vascularized from the very first. Part of the dedifferentiation of precartilage into reticulum consists of the invasion of blood-vessels into the precartilage region. In the early stages of the reticulum the larger vessels hug closely against the precartilage and continue to do so as the latter recedes from the epithelial duct, as can be seen in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 11, 12, and 14]]. Later, with the abrupt dedifferentiation of the remaining precartilage into reticulum, the larger vessels do not follow the receding margin of the cartilaginous canal, but form vascular arches that are suspeiulod in the reticulum, as can be seen in [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figures 15, 16, and 17]], and from these a network of small vessels branches toward the membranous duct on the one hand and the cartilaginous wall on the other. |
| precartilage, from precartilage to the tissue that is in transition to the reticulum and
| |
| then to the perichondrium. These are found as narrow zones that merge quickly
| |
| from one into the other. One should remember that the cartilaginous canal has not
| |
| reached its full size yet, and that the margin of the canal is still in an unstable condition. However, as the canal becomes larger and the tissues more mature, it is
| |
| found that the transitions between the different zones become more abrupt and
| |
| in this process the precartilage zone becomes relatively much narrower. This can
| |
| be seen by comparing text-figures 3 and 4. The width of the reticulum in these | |
| two figures can not be compared, because the.v represent diflferent canals, lateral
| |
| and posterior, and no attempt was made to take them from the same relative positions. The fact that the reticulum is narrower in figure 4 has no significance in | |
| the question of growth. The wide precartilage zone in figure 3 as compared with
| |
| that in figure 4, on the contrary, has a direct bearing on the relative age of the two
| |
| specimens. A relatively wide zone of precartilage is characteristic of younger
| |
| stages. After fetuses become 70 mm. long the precartilage zone becomes quite
| |
| narrow, so that the transition from cartilage to perichondrium is relatively abrupt.
| |
| In older si^ecimens one might easily obtain the impression that the perichondrium
| |
| rested directly against the cartilage, as doubtless it does in the adult. In the oldest
| |
| fetus examined, 130 mm. crown-rump length, there is still found a distinct though narrow precartilage-reticular transitional zone between the cartilage and the perichondrium. Presumably this indicates that the margin is still in an unstable
| |
| condition.
| |
|
| |
|
| After the perichondrium has
| |
| made its first appearance it rapidly becomes thicker and more
| |
| conspicuous. In a fetus 80 mmcrown-rump length (Carnegie Collection, No. 172) it is found as
| |
| quite a dense fibrous coat, more
| |
| than twice as thick as that shown
| |
| in the 73 mm. embryo in figure 4.
| |
| It is clearly separated from the
| |
| cartilage and precartilage by a
| |
| narrow zone of reticular tissue.
| |
|
| |
|
| The character of the perichondrium as existing in slightly
| | In [[Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7#Plate_1|figure 17]], which is a section through the posterior semicircular canal in a fetus 52 mm. crown-rump length (Carnegie Collection, No. 96), the reticulum is more mature in its appearance than any that have thus far been described. There is practically no precartilage to be seen. The reticulum now only lacks the membrane-like thickening of its inner and outer margins to render it complete. At the inner margin the cells arrange themselves into a fibrous coat that constitutes the membrana proipria of the membranous duct. At the outer margin is formed the perichondrium, the development of which will now be considered. |
| older fetuses is shown in figure 18,
| |
| which represents a section through | |
| the posterior semicircular canal of | |
| a fetus 85 mm. crown-rump length | |
| (Carnegie Collection, No. 140030). Here the perichondrium | |
| consists of a relatively broad
| |
| zone of enibrj'onic fibrous connective tissue, which in the photograph is about 5 mm. wide,
| |
| encircling the whole canal. It
| |
| can be seen on the median side
| |
| (to the left) that it is sejxirated
| |
| from the cartilage and adjacent
| |
| transforming precartilage zone by
| |
| a narrow, lighter area, which under higher magnification is found
| |
| to consist of reticular tissue. The membrana propria at the inner margin of the
| |
| reticulum is fairly well developed and it can be seen how it forms a supporting
| |
| coat to tho epithelial duct.
| |
|
| |
|
| \\'hen one examines the cartilaginous semicircular canals in fetuses 130 mm.
| |
| long there can no longer be any ([uestion as to the identitj' of the perichondrium.
| |
| A specimen showing the superior semicircular canal at this stage is represented in
| |
| figure 19, which is taken from a fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018). The blood-vessels are injected with India ink. The main cartilaginous mass in this specimen is (luite mature; the capsules are well defined and
| |
| the cartilage cells now possess a considerable amount of granular bodj'-protoplasm.
| |
|
| |
|
| | | {{Template:Carnegie No.20 Footer}} |
| | |
| Cartilage
| |
| | |
| | |
| | |
| Flu. 4. — Detail of the posterior canal in a human fetus 73 iniii. long
| |
| (Carnegie Collection, No. 1373, slide 0, row 3. section 1)'
| |
| The section is lOiU thick and is enlarged 370 diameters. It
| |
| shows how the inner margin ()f< the reticulum becomes con(hm.sed into the niembnina projiria of the epithelial duct and
| |
| the outer margin into I he iicricliondrium. The perichondrium
| |
| does not lie in direct cdiitact with the cartilage, hut is separated by a narrow zone of tissue which consists of precartilage,
| |
| into which the cartilage is still being dedifferentiated.
| |
| | |
| In many instances capsules are found containing more than one cartilage cell,
| |
| showing the tendency to cell columns.
| |
| | |
| A casual glance at a section under lower powers might indicate that the inner
| |
| maigin of the cartilage is in direct contact with the perichondrium. Examination
| |
| under higher magnification, however, shows that between the thick perichondrium
| |
| and the cartilage there is a narrow zone of dedifferentiated cartilage. In it the
| |
| matrix has largely disappeared and the capsules have collapsed and are flattened
| |
| out, allowing the elongated endoplasm of adjacent cartilage cells to come in contact, separated only by the remnants of the capsular margins. Dyes that stain
| |
| endoplasm red cause this zone to appear as a deep-red line. This zone represents
| |
| a state of transition between cartilage and precartilage and its presence doubtless
| |
| indicates that the margin of the cartilage is still in an unstable condition. The
| |
| narrowness of the zone and the abruptness of the transition are characteristic of
| |
| later stages, where the process is more gradual and relatively small in amount.
| |
| The transition from this zone to the perichondrium is likewise abrupt. The perichondrium consists of a dense protoplasmic stratum thickly studded with nuclei,
| |
| and has all the appearance of late embryonic fibrous connective tissue. It is of
| |
| about the same tliickness around the whole margin of the canal. At the outer
| |
| margin (toward the right) it fuses wdth the membrana propria of the epithelial
| |
| duct, therebj' forming an attachment which is regarded as a suspensory ligament
| |
| for the sujjport of the membranous labyrmth. The trabeculae of the reticulum
| |
| extending between the membrana propria and the perichondrium are just beginning to break apart, allowing the adjacent spaces of the reticulum, as they are seen
| |
| in section, to coalesce in the formation of larger spaces.
| |
| | |
| Having completed the review of the early history of the reticulum and its
| |
| formative relations to the adjacent tissues, we are now in a position to consider
| |
| the development and the fate of these larger spaces in the reticulum, which ha\'e
| |
| hitherto been generally known by the misleading term "perilymphatic spaces."
| |
| | |
| DEVELOPMENT OF PERIOTIC TISSUE— SPACES.
| |
| | |
| In the }3receding pages of this article the main features of the development of
| |
| the cartilaginous capsule that incloses the membranous labyrinth have been
| |
| described. We have traced the process step by step from the first condensation
| |
| of the mesenchjone around the otic vesicle, through its differentiation into a precartilaginous mass and the maturation of the latter into true cartilage, with the
| |
| formation through dedifferentiation of cartilaginous chambers in wliich the membranous labyrinth is suspended. It has been shown how these spaces within the
| |
| cartilaginous capsule are modified in adaptation to the continued growth of the
| |
| membranous labyrinth and how they finally come to be fiUed with an open-meshed
| |
| reticulum which everjT\ here bridges the space existing between the membranous
| |
| labyrinth and the surrounding cartilage. It has further been shown that the membrana propria supporting the epithehal part of the labyrinth on the one hand and
| |
| the perichondrium on the other are derived from and serve as the hmiting membranes of this reticulum. It is a modification in the meshes of this same reticulum that results in the formation of the so-called perilymphatic si)aces, or periotic spaces
| |
| as they will be referred to in this paper, the development of which will now he
| |
| outlined.
| |
| | |
| Thus far attention has been directed primarily to regions included in typical
| |
| transverse sections through the semicircular canals. This was done for the purpose
| |
| of uniformity and simplicity and because of the- ease with which successive stages
| |
| could be compared with one another. For studying the periotic spaces, however,
| |
| the region of the canals is not so favorable, because the spaces are late in developing
| |
| there, and even in their completed form they are not so well defined and highly
| |
| differentiated as those in the region of the vestibule and cochlea.
| |
| | |
| The earliest evidence of a periotic space makes its appearance opposite the
| |
| stapes. It is developed in the reticulum that fills the interval situated between
| |
| the saccule, utricle, and the cartilaginous stapes. Even before the general periotic
| |
| reticulum becomes very extensive, in embryos between 30 and 40 mm. long, it can
| |
| be seen that its meshes are more irregular and more open in this region than elsewhere. This is the rudimentary form of the periotic vestibular cistern, which is
| |
| the first space to become established.
| |
| | |
| DEVELOPMENT OF THE PERIOTIC CISTERN OF THE VESTIBULE.
| |
| | |
| Aside from the scala vestibuli and the scala tympani, the largest of the periotic
| |
| spaces is the large reservoir situated between the tympanic wall of the bony vestibule with its articulated stapes and the vestibular chambers of the membranous
| |
| labyrinth. This is the spatium perilymphaticum vestibuli (BNA) or the cisterna
| |
| perilymphatica (Retzius). In order to eliminate the word lymphatic from the
| |
| terminology it will be designated here as the cisterna periotica vestibuli, or less
| |
| formally the j^eriotic cistern. In this manner the descriptive term introduced by
| |
| Retzius is retained.
| |
| | |
| Before there is any trace of the scalse the initial steps in the formation of the
| |
| cistern can be seen. This is well illustrated in an embryo 35 mm. long (Carnegie
| |
| Collection, No. 199). This particular embryo is cut in a sagittal series and the
| |
| sections on slides 53 and 54 show the periotic cistern in its most rudimentary form.
| |
| It consists of an area of reticulum bounded by the utricle, saccule, ductus reuniens,
| |
| the proximal end of the cochlear duct, and the ampulla of the jiosterior semicircular
| |
| duct. The greater part of the periotic reticulum at this time (35-mm embryo) is
| |
| characterized by a narrow and uniform mesh that is interrupted only by numerous
| |
| cajjillaries branching through it; in the area mentioned, however, the spaces are
| |
| larger and are more irregular both in shape and in size. They i)resent the appearance seen along the semicircular ducts in considerably older embryos, for instance,
| |
| in the 52-mm. embryo, as is shown in figure 17. From the very first the increase in
| |
| the size of the mesh seems to be attained by the detachment and retraction of its
| |
| constituent i)rotoplasmic bridges, thereby allowing adjacent spaces to unite in the
| |
| formation of (•om])osite large spaces. Thus in the above section a few irregular
| |
| protopla.smic free-ends are seen still jjrojecting into the newly enlarged spaces.
| |
| This interesting histogenetic process will l)e taken up again later in connection with the development of the two scalae. The area of this rudimentary periotic cistern
| |
| is as yet very small and merges indefiniteh' into the adjoining reticulum. It is
| |
| not until we come to fetuses about 40 mm. long that it develops spaces of any considerable size, and it is not until we come to fetuses about 50 mm. long that we find
| |
| a single large space with walls that are definitely outlined, so that it can be satisfactorilj' modeled.
| |
| | |
| In a fetus 43 mm. long (Carnegie Collection, No. 886), which is cut in a coronal
| |
| series, the spaces forming the rudimentary cistern stand out much more definitely
| |
| than is the case in the 3o-mm. embryo that has just been referred to. There is
| |
| now just opposite the stapes one space which is much larger than the adjoining
| |
| spaces. On part of its margin the protoplasmic bridges are stretched along so as to
| |
| form a smoothly curved continuous boundarj-, which is defective in some portions,
| |
| and at such places the space merges with the adjoining secondary spaces. Within
| |
| the space are some fainth' refractive branching threads of coagulated plasma. The
| |
| scala vestibuli is not yet laid down and the scala tympani is only represented b}'
| |
| a moderate widening of the meshes of the reticulum in the neighborliood of the
| |
| fenestra cochleae (rotunda), along the basal border of the first turn of the cochlear
| |
| duct.
| |
| | |
| In fetuses 50 mm. long the outlines of the cistern become very distinct, due to
| |
| the marked increase in the size of its main cavity and to the more definite membrane
| |
| at its junction with the rest of the reticulum. Its form and relations are shown in
| |
| figures 26 and 27. Thej- represent a median and a lateral view of a wax-plate
| |
| reconstruction of this region in a human fetus 50 mm. long (Carnegie Collection,
| |
| Xo. 84). Onlj^ the main cavity is shown in the model. At certain places around
| |
| its borders the meshes of the reticulum are uniting into larger spaces and these in
| |
| turn are taken up by the main cavity as it advances into the new territory. These
| |
| smaller incomplete spaces were omitted in constructing the plates of the model.
| |
| The rule was adopted that only the spaces that were outlined by a membrane-like
| |
| border should be traced on the plates and included in the model. This rule was
| |
| adhered to in all the models of this series.
| |
| | |
| Figures 26 and 27 show that the periotic cistern in 50-mm. embryos consists
| |
| of a flattened, rounded, bursa-like cavity intervening between the stapes and the
| |
| lateral surface of the saccule and adjoining utricle. It extends forward to the
| |
| ijmpuUa of the lateral canal and upward to the beginning of the crus commune.
| |
| Posteriorly it crowds backward against the ductus reuniens, filling in the space
| |
| between the utricle, saccule, and the proximal end of the cochlear duct. Both on
| |
| its median and lateral surfaces there is no further opportunity for expansion except
| |
| as the vestibule itself enlarges. The deUcate membrane-like wall of the cistern
| |
| hugs closely against the parts of the membranous labyrinth on the one side and the
| |
| tympanic wall of the cartilaginous vestibule on the other, being separated from them
| |
| only by a thin layer of the original reticulum. Along the dorsal margin of the cistern, however, there is room for expansion, and the reticulum in this region shows
| |
| enlarging spaces in the process of uniting with the main cavity. On its ventral
| |
| margin, near the cochlea and extending along the apical surface of the latter, there is a definite row of reticular spaces actively coalescing and constituting the beginning of the scala vestibuli. These are shown in figure 21, which is a section of a
| |
| fetus of about the same age. The spaces of the scala vestibuli lie between the
| |
| cochlear duct and the cistern. This section also shows veiy well the relation of
| |
| the stapes to the cistern. The scala tymi)ani is already well started at this time,
| |
| but its development is quite independent of the cistern. Within the cistern can
| |
| be seen scattered clumi:)s of faintly refractive granular threads of what seems to be
| |
| a coagulated constituent of the plasma.
| |
| | |
| The subsequent growth of the cistern is shown in figures 28 to 31. Figures 28
| |
| and 29 show respectively a median and lateral view of a wax-plate reconstruction
| |
| of the membranous labyrinth and its periotic spaces in a human fetus 85 mm.
| |
| long (Carnegie Collection, No. 1400-30). The growth of the cistern here has kept
| |
| pace with the increase in size of the lab3'rinth and maintains the same general
| |
| relations as regards the stapes and the parts of the membranous labyrinth. The
| |
| view of the cistern in figure 28 is an oblique one which would tend to mislead
| |
| one as to its width. In reality it is relatively a little wider. It has also extended
| |
| upward on the dorsal surface of the utricle and is beginning to creep along the
| |
| inner side of the jjostcrior end of the lateral semicircular duct. Ventrally it communicates freely with the scala vestibuli, which now extends well down along the
| |
| cochlear duct.
| |
| | |
| The oldest stage studied is shown in figures 30 and 31. These show two views
| |
| of a wax-plate reconstruction of these structures in a human fetus 130 mm. long
| |
| (Carnegie Collection, No. 1018). At this time the periotic cistern has spread over
| |
| the vestibular part of the meml>ranous labyrinth, covering it nearly eveiywhere
| |
| excepting at the macular jiortions where the nerves terminate. In figure 31 it can
| |
| be seen that the mesial surface of the saccule is not covered ; this lies close against
| |
| the wall of the cartilaginous vestibule. The uppermost division of the cistern,
| |
| situated between the crus commune and the ampulla of the posterior semicircular
| |
| duct, does not yet open into the general cavit3^ It has formed separately and owing
| |
| to the i)osition in which it lies its coalescence with the other parts of the cistern is
| |
| retarded ; otherwise, free communication exists between all divisions of the cistern.
| |
| | |
| DEVELOPMENT OF THE PERIOTIC SPACES OF THE SEMICIRCULAR DUCTS.
| |
| | |
| From the descriptions given of the adult the reticulum along the ducts never
| |
| develops a single continuous wide periotic space like that of the cistern and the
| |
| two scala?. There always remain a few trabecular, such as are seen in the cistern
| |
| and scala? in their earlier stages, and these constitute partitions which traverse the
| |
| space and give it in sections the a])pearance of a series of sej^arate spaces extending
| |
| along the inner margins of the semicircular ducts. Although these spaces along
| |
| the ducts are inc()mi)lete as compaicd with the cistern and scahc, they are, however,
| |
| entirely analogous with them in their formation.
| |
| | |
| The space along the lateral semicircular duct is the largest. Its posterior end
| |
| exists as a continuation of the cistern. This can be seen in the lateral view of the
| |
| model shown in figure 30, where the cistern extends for a considerable distance along the inner border of the lateral duct. Along the other two ducts of the same
| |
| specimen (130 mm. crown-rump length) the reticulum has commenced the process
| |
| of space-formation, but complete channels are not yet established. A typical
| |
| section through one of the semicircular ducts in a fetus of this size, and this is the
| |
| oldest fetus studied, is shown in figure 23. As compared with the scalse in the
| |
| same fetus, as shown in figure 20, the space-formation along the ducts is very much
| |
| retarded.
| |
| | |
| DEVELOPMENT OF SCALA TYMPANI AND SCALA VESTIBULI.
| |
| | |
| The scala vestibuli may be regarded as an extension of the cistern downward
| |
| into the region of the cochlea and as such its growth starts from a focus opposite
| |
| the fenestra vestibuli (ovahs) . The scala tympani in a similar way makes its first
| |
| appearance opposite the fenestra cochleae. From these two foci the scalae extend
| |
| gradually downward along the cochlear duct as two separate spaces which do not
| |
| communicate with each other until they reach the tip of the duct, where there is
| |
| finallj^ developed a free opening between them known as the helicotrema.
| |
| | |
| In their formation they go through a series of histogenetic changes essentially
| |
| in the same manner that has been followed in the case of the formation of the cistern ; this (as we shall see) consists of the enlargement of the spaces of the periotic
| |
| reticulum that originally occupied this region, the enlargement being a result of
| |
| the disappearance of the protoplasmic bridges of the reticulum, whereby adjacent
| |
| spaces unite in the formation of composite larger spaces. This process continues
| |
| until there is a single continuous space extending down along the cochlear duct
| |
| representing each scala and at the margins of each of them there is developed a
| |
| membranous arrangement of the reticular cells which completely walls ofif the space
| |
| from the surrounding tissue. In these alterations in the reticular mesh and in the
| |
| formation of the surrounding membrane there is an active change in the form of
| |
| the reticular cells, which repeatedly adapt themselves to the new conditions. There
| |
| is no evidence to indicate that smy other cells take part in the formation of the scalae.
| |
| | |
| The first evidence of the formation, of scalae is found in fetuses about 40 mm.
| |
| long, which stage is a little later than the first appearance of the cistern. In a fetus
| |
| 43 nmi. crown-rump length (Cargnegie Collection, No. 886), along the proximal
| |
| part of the cochlear duct on its basal surface there is a distinct widening of the
| |
| meshes of the periotic reticulum. This is the beginning of the scala tj^mpani.
| |
| On the opposite side of the cochlear duct, where one would look for the scala vestibuh, the periotic reticulum retains its primitive appearance characterized by a
| |
| narrow and rather uniform mesh. Thus the scala tjTnpani makes its appearance
| |
| slightly in advance of the scala yestibuli — that is, if we regard the latter as distinct
| |
| from the cistern.
| |
| | |
| In fetuses 50 mm. long both the scala tympani and the scala vestibuli can be
| |
| plainly identified, although they are still very incomplete. A wax-plate reconstruction of them, rejiresenting their form and their relation to the membranous
| |
| labyrinth in a human fetus 50 mm. crown-rump length (Carnegie Collection, No.
| |
| 84), is shown in figures 26 and 27, being a median and a lateral view respectively.
| |
| | |
| In preparing this and tlie models shown in figures 28 to 31, it is to be remembered
| |
| that only those periotic spaces are included that were outlined by a membranelike margin. In the adjacent reticulum there are spaces that are actively coalescing and gradually uniting with the main cavity. No attempt, however, was
| |
| made to show such spaces in the models. From figures 26 and 27 it will be seen
| |
| that the scala tympani is larger and more advanced in its development than the
| |
| scala vestibuli. The hitter is in its earliest stage and consists of hardly more than
| |
| a row of enlarged reticular spaces which extend downward from the cistern along
| |
| the dorsal and apical surface of the cochlear duct. A section through the scala
| |
| vestibuli in another fetus of about the same age (Carnegie Collection, No. 448) is
| |
| roughly shown in figure 21, the spaces of the scala being situated between the
| |
| cistern and the cochlear duct.
| |
| | |
| The scala tympani consists of an elongated oval space lying along the basal
| |
| surface of the proximal jiart of the cochlear duct, about corresponding to the proximal half of the first turn of the duct. In the main part it is a single space with a
| |
| distinct margin separating it from the general periotic reticulum. In the more apical
| |
| portion it tapers off into multiple incompletely united smaller spaces which actively
| |
| coalesce as the process advances into the new territory along the duct. It is of
| |
| interest to note that the most mature and the largest part of this scala, representing
| |
| the focus at which it first appeared, is opposite the fenestra cochleae (rotunda),
| |
| just as the cistern forms opposite the stapes and the fenestra vestibuli. The scala
| |
| tympani alw-aj's begins at the same place and extends downward along the cochlear
| |
| duct, at first a Uttle in advance of the scala vestibuli, but subsequenth^ the latter
| |
| catches up with it and the two reach the tip of the duct at about the same time.
| |
| | |
| It is well known that the proximal portions of the cochlear duct mature sooner
| |
| than the distal portions. One might expect that the accompanying periotic spaces
| |
| would correspond in their development to the maturity of the duct and therefore
| |
| the proximal parts of the scalse would differentiate first. In other words, the
| |
| maturation of the cochlea proceeds as a wave from the proximal end to its tip,
| |
| involving all of its constituent structures as it passes along, including mesenchymal
| |
| j)arts as well as epithelial.
| |
| | |
| This conception might exi)lain the direction of development of the scalae, but can
| |
| hardly be ai:)plied to the cistern, the vestibular repres(>ntative of the scala vestibuli.
| |
| One can not say that those portions of the membranous labyrinth lying opi^osite the focus of development of the cistern (that is, the lateral walls of the saccule
| |
| and utricle) mature in advance of the rest of the labyrinth. There is no indication
| |
| that a wave of differentiation passes through the epithelial elements of the labyrinth in the same direction and sj'nchronously with the extension of the cistern
| |
| as it advances from its j)rimary focus u])()n the roof of the utricle and over on its
| |
| median surface. In the case of the ci.stern it seems much more likely that the point
| |
| at which it first apjiears is determined by the position of the stai)es, which is doubtless an expression of the physical relation that subsequently exists between the two.
| |
| Hy analogy this would yield additional significance to the relation existing between
| |
| the fenestra cochlea; and the point of beginning development of the scala tympani.
| |
| | |
| In dealing with the cistern and also with the scalse one should not consider
| |
| them as insignificant accessories that merely fill in the waste intervals between the
| |
| membranous labyrinth and the surrounding cartilage. From stud3dng their development it becomes apparent that they have a morphological individuality in many
| |
| respects as definite as that of the ossicles themselves. They make their appearance at a definite time and at definite places, they spread in a definite manner, and
| |
| eventually they attain a form and structure that are adapted to a definite function.
| |
| This becomes more and more evident as we examine older stages.
| |
| | |
| The form and relations of the scalie in fetuses between 12 and 13 weeks old are
| |
| shown in figures 28 and 29. These figures show median and lateral views of a waxplate reconstruction of the membranous labyrinth and the surrounding periotic
| |
| spaces in a human fetus 85 mm. crown-rump length (Carnegie Collection, Xo.
| |
| 1400-30). Attention has already been directed to these figures in the description
| |
| previously given of the cistern. The scala vestibuli can be seen in figure 28. Above,
| |
| it opens freely into the cistern and extends downward along the apical side of the
| |
| duct as a single main space, possessing a rather uniform diameter. It extends
| |
| along the first two turns of the duct, gradually tapering off and showing a less
| |
| mature character in its distal portions. Along the second turn of the duct the
| |
| spaces are incompletely fused and the contour becomes correspondingly irregular.
| |
| As a rule the peripheral margin of the scala is less mature and more irregular than
| |
| the central margin. The scala, vestibuli does not connect with the scala tjTiipani
| |
| at any point as yet. The two are separated in the first place by the cochlear duct
| |
| and then more centrally b}- a framework of connective tissue in which are the
| |
| radiating bundles of the cochlear nerve with the nodes of ganglion cells that form
| |
| the spinal gangUon. These latter structures are not shown in the model; they
| |
| occupy, however, the V-shaped groove seen between the two scalae.
| |
| | |
| The scala tj^mpani, as can be seen in figure 29, extends downward on the basal
| |
| side of the cochlear duct along its first two turns. This corresponds to about the
| |
| same linear dimension as that of the scala vestibuli. In its proximal portion it
| |
| shows a greater area in cross-section than the latter, but further toward the apical
| |
| region it is of about the same size and in some places it is even smaller. The peripheral margin of the scala tympani is distinctly more irregular than the central
| |
| margin. The irregularity is due to spaces along this margin that are actively
| |
| coalescing with the main sjiace, but in which the fusion is not yet complete. The
| |
| irregularity of this margin is thus an indication of the direction of the expansion of
| |
| the scala. As the diameter of the whole cochlear mass increases, it is evident that the
| |
| main growth of the scala must radiate outward in a peripheral direction. This is
| |
| accomplished by the continual assimilation of new reticular spaces along this margin.
| |
| At the proximal end of the scala tympani can be seen an oval depression which
| |
| corresponds to the fenestra cochleie (rotunda) and with which it stands in intimate
| |
| relation.
| |
| | |
| In fetuses about 16 weeks old the form and relations of the scalae have nearly
| |
| attained the adult conditions, and this represents the oldest stage studied in connection with the present paper. The conditions found at this time are shown in figures 30 and 31, which present nuMhan and lateral views of a wax-plate model
| |
| of a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018).
| |
| On comparing the scala tympani and scala vestibuli as seen in these figures with
| |
| those in figures 28 and 29, it will be seen that they are larger in cross-section and
| |
| more nearlj- cover the cochlear duct. Furthermore, they now extend to the extreme
| |
| tip of the duct and communicate with each other across its central margin, thus
| |
| forming a helicotrema. A section through this point can be seen in figure 25, in
| |
| which these structures are shown as seen under low magnification. It will be
| |
| noted that now, even as far as the tip of the cochlea, each of the scalse consists of
| |
| a continuous principal space, though both are more mature and larger in their
| |
| proximal portions. Along the first turn of the cochlear duct they are walled off
| |
| by a smooth membranous margin which separates them from the adjacent reticular
| |
| tissue. The spaces of the latter do not seem to be taking anj^ further part in the
| |
| I)rocess of enlargement of the scala". Along the second turn of the cochlear duct,
| |
| a section of which is shown in figure 20, the coalescence of reticular spaces w'ith
| |
| each other and with the scalse is still in active operation. This produces a greater
| |
| irregularity of the scalar than is shown in the model. The subsidiary spaces are
| |
| shown as a solid mass; the slender clefts separating them are not represented. The
| |
| nearer one a})proaches the tip of the duct the more immature are the scalse, until
| |
| the condition is reached that is shown in figure 25, where the membrane-like margin
| |
| is {[uite incomplete and the spaces merge irregularly with the surrounding reticulum.
| |
| Thus a single specimen, if studied in its different parts, shows several stages in this
| |
| interesting process of the formation and growth of the scalaj.
| |
| | |
| The figures grouped on plate 3 illustrate some of the histological features
| |
| of this process. An early stage in space-formation is shown in figure 23. This
| |
| is a section through the canal region where the changes in the reticulum are late
| |
| in making their appearance. In fact, the periotic spaces never reach the same
| |
| degree of differentiation here that occurs in the case of the cistern and scalse. The
| |
| initial steps, how-evcr, are the same, and this figure presents very w^ell the appearance of the periotic reticulum as it begins to open up into larger spaces. Unmodified reticulum is characterized by a rather uniform narrow mesh. The essential
| |
| change in space-formation consists in the disappearance of some of the trabeculse
| |
| of the mesh, with the consequent coalescence of the corresponding adjacent spaces.
| |
| The trabecuhe consist of the protoplasmic processes of the constituent cells of the
| |
| reticulum and their disappearance is to be explained in either of two ways: It is
| |
| I)ossiblc that owing to some property of the fiuid element of the tissue the protoplasmic strands are dissolved or liquefied; this would account for their complete
| |
| disajipearance. On the other hand, the same result could be accomj)lished by an
| |
| alt(.'ration in the form of the cell processes. A given trabecula could separate at
| |
| either end, or at some jioint along its line, and the free ends of protoplasm could
| |
| then retract and reshape themselves and become a part of the remaining framework. Whether we are dealing with a licjuefaction of tissue or with active motility
| |
| of the cell, protoplasm involviiig detachment and retraction of the trabeculae can not be definitel}^ determined by observations of fixed tissue; but the appearance
| |
| of sections where the process is in active operation seems to the writer to indicate
| |
| the latter.
| |
| | |
| In the above paragraph and elsewhere in this paper reference is made to
| |
| trabeculse ser^dng as "partitions" between "spaces" and the disappearance of
| |
| trabeculse resulting in the "coalescence of adjacent spaces." In making this use
| |
| of the term "space" it should be explained that it is done in a descriptive sense, in
| |
| application to the appearance of the tissue as seen in sections in which form human
| |
| embryological material is mainly available. In thin sections of a reticular tissue
| |
| one sees trabecule as partitions separating adjacent spaces. The same tissue in
| |
| a mass would show that the spaces everywhere communicate freely with each other,
| |
| hke the spaces in a sponge, and that the trabeculse are thread-like strands which
| |
| at the best are very incomplete partitions. Instead of a meshwork containing
| |
| manj' small spaces, one could perhaps equalh' well describe reticular tissue as a
| |
| single large space traversed by mam^ trabeculse. If the latter practice were adopted,
| |
| one would describe the development of the tissue-spaces with which we are concerned as a process of gradual decrease in the number of traversing trabeculse,
| |
| with the result that the mesh thereby becomes coarser. For descriptive purposes,
| |
| however, it is convenient to refer to the intervals between the strands of the mesh
| |
| as spaces, at the same time not granting them the significance that is attached to
| |
| such membrane-Uned tissue-spaces as are represented by the vestibular cistern
| |
| and the two scalse, though the latter are in reaUty derived from them.
| |
| | |
| In figure 23 the free detached ends of the trabeculse will be noted everywhere,
| |
| as is characteristic of this stage of development. It is a necessarj^ step in the coalescence of adjacent spaces. The detached trabeculse seem to be gradually retracting
| |
| and adapting themselves to the formation of larger spaces. Their constituent
| |
| protoplasm reshapes itself as a smooth border or as a part of other trabeculse.
| |
| Larger spaces necessitate longer trabeculse, and as trabecuUe become longer they
| |
| also tend to become heavier. These phenomena are all in evidence in the spreading
| |
| and enlargement of the scalse.
| |
| | |
| Figure 20 shows a characteristic view of the scalse as seen under low magnification. It will be noted that the scala vestibuli is relatively mature at this
| |
| point; the scala tympani, however, is in the act of spreading peripherally, so as to
| |
| underUe, as it eventually will do, the future basilar membrane. The scala tympani
| |
| finally reaches the peripheral margin of the cochlear duct, and it does this by the
| |
| coalescence of the enlarging reticular spaces, which become incorporated with the
| |
| main cavity of the scala. This can be observed better in figure 22, which shows a
| |
| detail of the same section as seen under higher magnification. By comparing this
| |
| figure with figure 20 the exact location can be readily made out. A portion of the
| |
| main cavity of the scala is indicated and to the right of this are a few enlarged
| |
| reticular spaces that are uniting with each other and will in the end become part
| |
| of the main space. In adcUtion to the enlarged reticular spaces there is a certain
| |
| amount of residual undifferentiated reticulum. It is this tissue that will play the
| |
| part of an adventitial coat to the completed scala. The trabecula? that separate
| |
| the enlarged spaces seem to be under tension and about ready to snap apart. In
| |
| fact, in most sections one can see the fragmentary ends of trabeculse where this
| |
| interruption of continuity has apparently occurred.
| |
| | |
| The differentiation of the margin of the scalse constitutes the final feature in
| |
| their maturation. During the i)eriod in which the enlargement of an individual
| |
| scala is being brought about by the coalescence f)f enlarging reticular spaces, the
| |
| margins of the main cavity can be seen to consist of smooth, delicate strands of
| |
| nucleated protoplasm that resembles in all essentials that of the trabeculae between
| |
| the large reticular spaces. These linear margins are interrupted here and there
| |
| by openings into adjacent spaces, but they tend to form a continuous line that
| |
| definitely marks off the space from the adjacent reticulum. An early stage in the
| |
| formation of such a margin is shown in figure 25, where the margin is indicated at
| |
| a few places, but for the most i)art the space abuts against the surrounding ragged
| |
| reticulum. The margin of the space is more complete in the scala tympani shown
| |
| in figure 22, but it is still thin and delicate and can be easil}^ opened up to allow the
| |
| taking in of new spaces. If we examine the borders of more mature spaces we find
| |
| them inclosed by a firmer membrane, which finally reaches a state that will probably
| |
| not admit of any further oi)ening up for the coalesence of additional spaces. Any
| |
| further growth must thereafter be limited to simple distention of the wall of the
| |
| space with the consequent adjustment of its constituent cells. Such a condition
| |
| is represented in figure 24. This shows a more mature section of the wall of the
| |
| scala vestibuli, being a detail of the same section shown in figure 20. The only
| |
| difference between such a membrane, as we must now call it, and the corresponding
| |
| structure in younger stages is its density; it is wider and its protoplasm perhaps
| |
| more opaque, or in other words, more protoplasm is accumulated there.
| |
| | |
| If figures 24, 22, 25, and 23 are comi^aied and followed in that order, it
| |
| will be seen that the lining m('ml)rane of the scala* can be traced backward, step
| |
| by step, to the ordinary trabeculie of the periotic reticulum. There is no histological evidence that any new cells enter into its formation. It seems to be simply
| |
| a product of the proliferation and adaptive reshaping of the cells already there.
| |
| In its final form the margin of the space r(\seml)les an endothelial membrane. One
| |
| could describe, as inunediately lining the s])ace, a thin membrane with flattened
| |
| nuclei, which is sui)i)orted underneath by a thin coat of nucleated protoplasm thai,
| |
| has the form of fibrous connective tissue. The former, judging only from its final
| |
| aj)pearance, one might designate as endothelium and thus make a distinction between it and the underl^ying tissue. In its histogenesis, however, it differs in no way
| |
| from the rest of the wall and the difference that exists later seems to be merely the
| |
| result of its adaptation to the existing physical conditions. Its early l)ehavior is
| |
| entirely different from that of vasculiu- endothcliuiu. Thus if its final ajipearance
| |
| is stres.sed and the term endothelium is used for its designation, il must be done
| |
| with a considerable amount of reservation. It is preeminently a i)lace where the
| |
| term mesothelium could be used with great advantage.
| |
| | |
| COMMUNICATION OF PERIOTIC SPACES WITH ARACHNOID SPACES.
| |
| | |
| The relation of the scala tj^mpani and scala vestibuli to the subarachnoid
| |
| spaces surrounding the hind-brain is of considerable interest, both on account of
| |
| the possibiUty of their functional relationship and on account of the similarity that
| |
| exists in their development. For a satisfactory investigation of the establishment
| |
| and the character of the communications that are formed between these two allied
| |
| systems of tissue-spaces, one should resort to other methods than those used in
| |
| the present study, and, furthermore, one should examine older fetuses than those
| |
| described here. In fact, a problem lies here that would be well worth careful study.
| |
| | |
| Certain observations, however, were made in the course of the above investigation that bear relation to these matters, and they will be briefly outlined here.
| |
| In the first place, the histological picture of the periotic reticulum is essentially the
| |
| same as that of the early stages of the pia-arachnoidal tissue investing the central
| |
| nervous system. The enlargement of the meshes of the latter and the formation
| |
| of the subarachnoid spaces and the arachnoid cistern, as has been recently described
| |
| by Weed (1917), correspond exactly with the appearance seen in the histogenesis
| |
| of the periotic spaces in the ear. The periotic spaces are not, however, extensions
| |
| of the arachnoid spaces that have invaded the cavity of the cartilaginous labyrinth.
| |
| If this were so we should find them first appearing among the rootlets of the vestibular and cochlear nerves, along which the .subarachnoid space extends for some
| |
| little distance. Instead, they begin at points where there can be no connection
| |
| with the arachnoid tissue and their direction of growth is quite independent of it.
| |
| The periotic spaces maj' be analogous to the arachnoid spaces, but they are not
| |
| identical with them, nor are they an extension of them.
| |
| | |
| According to the descriptions of the adult anatomy of the ear, a communication becomes established between the scala tympani and the subarachnoid space
| |
| near the fenestra cochleae, the so-called aquaeductus cochleae. Vague and conflicting statements are also made concerning a communication through the internal
| |
| auditory meatus connecting the arachnoid spaces with the scalae. Such communication must be estabhshed quite late. In the oldest fetus examined, 130 mm.
| |
| crown-rump length, they did not yet exist. As to the latter communication, it
| |
| can be seen that the arachnoid spaces extend peri])herally through the internal
| |
| auditory meatus along the trunk of the acoustic nerve-comjilex, and slender pockets
| |
| and clefts from them extend along the larger bundles of the cochlear nerve; they
| |
| terminate, however, before reaching the margins of the scalae, and there is no evidence at this stage that there is ever to be a conununication between them and the
| |
| scalae. As to the aquaeductus cochleae, in the 130 mm. fetus it can be plainly seen
| |
| that it is already forming as a deri\'ative of the arachnoid spaces, although the communication with the scala tympani is not yet established. The arachnoid spaces
| |
| invest the glossojiharyngeal nerve and extend down along its trunk and pa.ss directly
| |
| V)y the region of the fenestra cochleae (rotunda). A thin-walled tubular pouch
| |
| projects from these spaces, leaving the nerve trunk and extending obliquely toward
| |
| the scala tympani in a direction that would meet it just distal to the fenestral impression on its basal surface. This fundament of the aqua?ductus cochleae is
| |
| present in fetuses 85 mm. crown-rump length, but is longer in the 130 mm. fetus,
| |
| where it nearly reaches the scala tympani. The communication must be established soon after this.
| |
| | |
| | |
| | |
| SUMMARY.
| |
| | |
| The changes in size and form which the cartilaginous capsule of the ear undergoes during its development in the human embryo are accomplished in part by a
| |
| progressive and in part by a retrogressive differentiation of its constituent tissues.
| |
| Throughout the entire period of growth, as far as material was available for study,
| |
| it was foimd that the margins of the cartilaginous cavities undergo a process of
| |
| continual transformation. They exhibit a state of unstable equilibrium in respect
| |
| to the opposing tendencies toward a deposit of new cartilage on the one hand and
| |
| toward the excavation of the old on the other. The margins therebj^ are always
| |
| either advancing or receding, and it is in this way that the progressive alterations in
| |
| the size, shape, and position of the cavities are produced, due to which a suitable
| |
| suite of chambers is always provided for the enlarging membranous labyrinth.
| |
| | |
| The general tissue mass of the otic capsule, during the period represented by
| |
| embryos from 4 to 30 mm. long, passes through three consecutive histogenetic
| |
| periods, nameh', the stage of mesenchymal syncj'tium, the stage of precartilage,
| |
| and the stage of true cartilage. In the subsequent growth of the capsule it is found
| |
| that in areas where new cartilage is being deposited the tissues of the areas concerned follow a definite and progressive order of development. In areas, however,
| |
| where excavation occurs, where cartilage previously laid down is being removed,
| |
| it is found that the process is reversed. The tissue in such areas returns to an
| |
| earlier embryonic state — that is, it undergoes dedifferentiation. Tissue that has
| |
| accjuired all the histological characteristics of true cartilage can thus be traced in
| |
| its reversion to precartilage and from jirecartilage in turn to a mesenchymal syncytium. In the latter form it redifferentiates into a more specialized tissue, in
| |
| this case for the most part into a vascular reticulum.
| |
| | |
| The formation of the periotic reticulum is first indicated by a cluster of deeply
| |
| staining nuclei that can be seen along the central edge of the semicircular ducts in
| |
| embryos soon after the ducts are formed, and at about the time the otic capsule
| |
| begins to change from condensed mesenchyme into precartilage. These nuclei
| |
| constitute a focus at which the development of the reticulum and its blood-vessels
| |
| takes origin. Here the tissue of the otic capsule takes on an appearance that is
| |
| less like that of a cartilage-forming tissue and more like that of an embryonic connective tissue. Spreading from this focus, a narrow area is established which soon
| |
| encircles the semicircular ducts and becomes the open-meshed vascular reticulum
| |
| which, in embryos 30 mm. long, everywhere bridges the space existing lietween the
| |
| epithelial lal)yrinth and the surrounding cartilage. In the earlier stages it could
| |
| not be definitely shown that the primordium of the i^eriotic reticular tissue is not
| |
| derived from a few ])rcdestined mesenchyme cells which become inclosed, along with
| |
| the otic vesicle, by the condensed tissue of the capsule and after a certain latent period undergo proliferation and occup}' the space vacated by the receding precartilage. In the later stages, however, it is cjuite evident that precartilage tissue
| |
| is actually' converted into a reticulum, and that the replacement of precartilage
| |
| by a reticular connective tissue is brought about through a process of dedifferentiation.
| |
| | |
| The perichondrium is a derivative of the periotic reticulum and forms an outei
| |
| limiting membrane along its cartilaginous margin. During the fetal period the
| |
| perichondrium does not rest directly against the true cartilage, but is separated from
| |
| it by a zone of transitional tissue consisting partly of precartilage and partly of
| |
| reticulum. This transitional zone intervening between the perichondrium and the
| |
| surrounding cartilage was ob-served in all of the specimens that were studied, which
| |
| includes fetuses up to 130 mm. crown-rump length. Owing to the fact that the
| |
| perichondrium is late in making its appearance, being first seen in fetuses about
| |
| 70 mm. long, it can take no part in the early changes in the cartilaginous capsule,
| |
| either as regards the deposit of new cartilage or the excavation of cartilage that
| |
| had been previously laid down.
| |
| | |
| The periotic tissue-spaces are formed by a modification of the meshes of the
| |
| periotic reticulum. The latter consists originally of a rather uniform narrow mesh.
| |
| The essential change which it undergoes in the process of space-formation consists
| |
| in the gradual disappearance of the traversing trabeculse. The trabeculae consist
| |
| of the protoplasmic processes of the constituent cells of the reticulum, and their
| |
| disappearance is apparently due, not to a dissolution or Uquefaction of these cellprocesses, but to an alteration in their form. It apparently is the result of an active
| |
| motility of the cell protoplasm involving the successive detachment and retraction
| |
| of the trabeculse. Wlien a trabecula becomes detached it retracts and adapts
| |
| itself to the formation of the enlarging space, reshaping itself either as a smooth
| |
| border or as a constituent part of another trabecula.
| |
| | |
| The differentiation of the margin of the periotic spaces constitutes the final
| |
| feature in their maturation. During the period in which the enlargement of an
| |
| individual space is activeh' going on,. the margins of the main cavity consist of
| |
| smooth, delicate strands of nucleated protoplasm that resemble the trabeculse
| |
| between the large reticular spaces. These linear margins are interrupted here and
| |
| there by openings into adjacent spaces. They tend, however, to form a continuous
| |
| hne that definitely marks ofT the space from the adjacent reticulum. As the space
| |
| becomes more mature, the membrane-like border becomes thicker until it reaches
| |
| a state that will probably not admit of any further opening-up for the coalescence
| |
| of additional spaces. Any further growth is thereafter limited to a simple distention
| |
| of the wall of the space, with the consequent adjustment of its constituent cells.
| |
| In its final form the margin of the space constitutes a mesothehal membrane.
| |
| Immediately lining the space is a thin membrane with flattened nuclei which is
| |
| supported underneath by a thin coat of nucleated protoplasm having the form of
| |
| fibrous connective tissue. The former in its histogenesis differs in no way from
| |
| the rest of the wall and the difference that exists later seems to be merely the
| |
| result of its adaptation to the existing physical conditions.
| |
| | |
| 'J'hc oarlie.st histological evidence of the formation of the periotic spaces occurs
| |
| near the stapes, in the reticulum that bridges the interval l)etween the sacculus
| |
| and the fenestra vestibuh. In embrj'os between 30 mm. and 40 mm. long, it can
| |
| be seen that the meshes in this region are becoming irregular and larger, due to the
| |
| disapjiearance of some of the trabeculae and a consequent coalescence of the intertrabecular si)aces. The widening of the mesh at this point constitutes the primordium of the vestibular cistern. It makes its appearance before there is any trace
| |
| of the scalse, but it is not until the fetus reaches a length of about 50 mm. that the
| |
| cistern becomes definitely outlined and clearly differentiated from the adjoining
| |
| reticulum.
| |
| | |
| Following the appearance of the cistern, the scala tympani is the next space to
| |
| become established. It can be recognized as a moderate widening of the meshes
| |
| of the reticulum in the region of the fenestra cochleae in fetuses 43 mm. long, along
| |
| the basal border of the first turn of the cochlear duct. The scala vestibuli, as can
| |
| be seen in fetuses 50 mm. long, develops as an extension downward of the cistern
| |
| along the apical border of the cochlear duct. Starting from these definite foci,
| |
| these three spaces spread into their destined territory, absorbing as they go the
| |
| enlarging reticular spaces of the invaded region by a process of space-coalescence,
| |
| or, in other words, the progressive formation of areas that are free of trabeculse. In
| |
| fetuses 85 mm. long the two scalse extend downward along the cochlear duct to
| |
| its last turn, as two separate spaces which do not communicate with each other.
| |
| When they reach the tip of the duct, which occurs in fetuses about 130 mm.
| |
| crown-rump length, a free opening is developed between them which represents
| |
| the hehcotrema. After being completely established along the whole length of
| |
| the cochlear duct, the scalae continue to enlarge by further coalescence of tissue along
| |
| their peripheral border, in which the trabecular disappear.
| |
| | |
| The periotic sjxices are analcjgous in their development to the pia-arachnoidal
| |
| spaces; they are not, however, extensions of them that have invaded the cavity of
| |
| the cartilaginous labyrinth. They begin at points where there can be no connection with the arachnoidal tissue and their direction of growth is quite independent
| |
| of it. The communication that is found in the adult between the scala tympani
| |
| and the subarachnoid sjjace in the neighborhood of the fenestra cochleae, the socalled af|ua'ductus cochleae, is established (juite late. In fetuses 85 mm. crownrump length it exists as a tubular pouch projecting from the subarachnoid s])aces
| |
| along the glossopharyngeal nerve toward the scala tj^mpani. In the 13()-mm. fetus,
| |
| the (jldest examined, this j^ouch is longer and nearly reaches the scala. The communication must be established soon after this.
| |
| | |
| Similar iirojections from the subarachnoid spaces at the internal auditory
| |
| meatus extend as perineural clefts along the trunk and branches of the acoustic
| |
| nerve. No actual cf)mnnuiications, however, were seen between these spaces and
| |
| the two scalae.
| |
| | |
| | |
| | |
| BIBLIOGRAPHY.
| |
| | |
| | |
| | |
| Balfocr, F. M., 1885. A treatise on comparative em
| |
| brj'ologj'. 2 ed., vol. 2. London.
| |
| Bardeen, C. R., 1910. Die Entwicklung des Skeletts
| |
| und des Bindegewebe.s. Keibel-Mall, Handbuch
| |
| | |
| der Entwicklungsgeschichte, i. 296-456.
| |
| BoETTCHER, A., 1869. Ueber Entwiokelung und Bau des
| |
| Gehorlabyrinths nach Untersuchungen an Saugethieren. Dresden. (Quoted from Retzius, 1884.)
| |
| , 1872. Kritische Bemerkungen und neue Beitrage ziir Literatur des Gehorlabyrinths. Dorpat.
| |
| (Quoted from Retzius, 1884.)
| |
| Breschet, G., 1836. Recherches anatomiques et physi
| |
| ologiques sur I'organe de I'ouie et sur I'audition
| |
| | |
| dans I'homme et les animau-x vertebres. 2 ed.
| |
| | |
| Paris.
| |
| Cassebohm, J. F., 1735. Tractatus anatomici de auie
| |
| | |
| humana, etc. Halae, Magdebiu-gica?. (Quoted
| |
| | |
| from Breschet, 1836.)
| |
| Child, C. M., 1915. Senescence and rejuvenescence.
| |
| | |
| University of Chicago Press.
| |
| CoRTi, A., 1851. Recherches sur I'organe de I'ouie des
| |
| | |
| mammiferes. Premiere partie. Zeitschr. f. wiss.
| |
| | |
| Zool.. Ill, 109-169.
| |
| CoTUNNn, D., 1760. De aqujeductibus auris humaris
| |
| | |
| intemae anatomica dissertatio. NapoU. (Quoted
| |
| | |
| from Breschet. 1836.)
| |
| vox Ebner, v., 1902. Kolliker's Handbuch der Gewe
| |
| belehre. Bd. in. 2, 6 .\ufl.
| |
| FiLATOFF, D., 1906. Zur Frage iiber die Anlage des
| |
| | |
| Knorpelschadels bei einigen Wirbeltieren. ."Vnat.
| |
| | |
| Anz., XXIX, 623-633.
| |
| Gaupp, E., 1906. Die Entwickelung des Kopfskelettes.
| |
| | |
| Hertwig's Handbuch der Entwick . d. Wirbeltiere.
| |
| H.\sse, C, 1873. Die Lymphbahnen des inneren Ohres
| |
| | |
| der Wirbelthiere. In his Anatomische Studiem.
| |
| | |
| Leipzig., I, Heft 4, Xo. 19, 766-816.
| |
| , 1881. Bemerkungen iiber die Lymphbahnen des
| |
| | |
| inneren ohres. Arch. f. Ohrenh., xvii, 188-194.
| |
| Hensen, v., 1863. Zur Morphologic der Schnecke des
| |
| | |
| Menschen und der Saugethiere. Ztschr. f. wiss.
| |
| | |
| Zool., XIII, 481-512.
| |
| His, W., 1903. Die Haute und Hohlen des Korpers.
| |
| | |
| Wiederabdruck eines akademischen Programmes
| |
| | |
| vom Jahre 1865. Arch. f. Anat. u. Physiol., Anat.
| |
| | |
| Abth., 368-404.
| |
| Huschke, E., 1831 . Erste Bildungsgeschichte des Auges
| |
| | |
| und Ohres. (Vereammlung Naturforscher u.'
| |
| | |
| Aerzte zu Hamburg.) Isis von Oken, 950.
| |
| Key, E. a. H., and G. Retzius, 1875-1876. Studien in
| |
| | |
| der Anatomic des Xer\-ensy stems und des Binde
| |
| gewebes. Stockholm.
| |
| KoLLiKER, A., 1850-1854. Mikroskopische Anatomic
| |
| | |
| oder Gewebelehre des Menschen. Leipzig.
| |
| , 1861. Der embrj-onale Schneckenkanal und
| |
| | |
| seine Beziehungen zu den Theilen der fertigen
| |
| | |
| Cochlea. Wiirzburger natiirwiss. Zeitschr., ii, 1-9.
| |
| , 1861. Entwicklungsgeschichte des Menschen
| |
| | |
| luid der hoheren Thiere. Leipzig.
| |
| , 1879. Entwicklungsgeschichte des Menschen und
| |
| | |
| der hoheren Thiere. 2 ed., Leipzig.
| |
| , 1884. Grundriss der Entwicklungsgeschichte des
| |
| | |
| Menschen und der hoheren Thiere. 2 ed., Leipzig.
| |
| Krause, R., 1906. Entwickelungsgeschichte des Gehor
| |
| organes. Handbuch d. vergl. u. expcr. Entwick
| |
| lungslehre der Wirbeltiere. HeravLsg. v. O. Hert
| |
| wig. Jena.
| |
| Levi, G., 1900. Beitrag zuin Studium der Entwiekelung
| |
| | |
| des Knorpehgen Primordial craniums des Menschen. Archiv f. mikrosk. Anat., Bd. 55
| |
| | |
| | |
| | |
| Lewis, W. H., 1907. On the origin and differentiation of
| |
| the otic vesicle in amphibian embryos. Anat.
| |
| Rec, I, 141.
| |
| | |
| , 1915. The use of guide planes and plaster of
| |
| | |
| paris for reconstruction from serial sections : Some
| |
| points on reconstruction. Anat. Rec, ix, 719.
| |
| | |
| Macklin', C. C, 1914. The skull of a human fetus of
| |
| 40 mm. .A.nier. Jour. .\nat., vol. 16.
| |
| | |
| Mall, F. P., 1902. On the development of the connective tissues from the connective tissue syncytium.
| |
| Amer. Jour. Anat., i, 329-365.
| |
| | |
| Morgagni, J. B., 1740. Epist. anatom., xii, p. 469.
| |
| Venetiis. (Quoted from Breschet, 1836.)
| |
| | |
| Odenius, M. ^'., 1867. Ueber das Epithel der Maculae
| |
| acusticae beim Menschen. .Arch. f. mikr. Anat.,
| |
| Ill, 11.5-134.
| |
| | |
| Reichert, C. B., 1852. Bericht iiber die Abhandl. d.
| |
| Herm Dr. Reissner "De auris interns formatione." Bull. d. la Cl. phys.-math. d I'acad. imp.
| |
| d. sci. d. St. Pgtersbourg, x, 86-96.
| |
| | |
| , 1864. Beitrag zur feineren Anatomie der Gehor
| |
| schnecke des Menschen und der Saugethiere.
| |
| .\bhandl. d. Kgl. .\kad. d. WLss., Berlin, (Phvs.)
| |
| Xo. 1, 1-60.
| |
| | |
| Reissner, E., 1851. De auris internseformationc. Inaug.
| |
| diss., Dorpat. (See Reichert, 1852.)
| |
| | |
| , 1854. Zur Kenntniss der Schnecke im Gehor
| |
| organ der Saugethiere und des Menschen. J. Midler's Arch. f.*.\nat., Physiol, u. wiss. Med.420-427.
| |
| | |
| Retterer, E., 1900. Evolution du cprtilage transitoire.
| |
| Jour, de I'Anat. et de la Physiol., xxxvi, 467-565.
| |
| | |
| Retzius, G., 1884. Das Gehororgan der Wirbelthiere.
| |
| Abtheilung ii. Stockholm.
| |
| | |
| Sabin, F. R., 1917. Origin and development of the
| |
| primitive vessels of the chick and of the pig. Contributions to Embrj-ologj', vol. 6, No. 15. Carnegie Inst. Wash. Pub. No. 226.
| |
| | |
| Scarpa, A., 1789. Anatomicae disquisitiones de auditu
| |
| et olfactu. Ticini. (Quoted from Retzius, 1884.)
| |
| | |
| ScHWALBE, G., 1869. Der Arachnoidalraum ein Lymphraum und sein Zusammenohang mit dem Perichorioidalraum. Centralbl. f. d. med. Wiss., No.
| |
| 30, 465-467.
| |
| | |
| SoLGER, B., 1889. Ueber Knorpelwachstum. Verb. d.
| |
| Anat. Gesellsch. BerUn, in, 67-71. (Erganzungsheft Anat. Anz., rv.)
| |
| | |
| Streeter, G. L., 1917. The factors involved in the
| |
| excavation of the cavities in the cartihginous capsule of the ear in the human embiyo. Amer.
| |
| Jour. Anat., xxii, 1-25.
| |
| | |
| , 1917. The development of the scala tympani,
| |
| | |
| scala vestibuU, and perioticular ci.stern in the
| |
| human embryo. .\mer. Jour. .Anat., xxi, 299-320.
| |
| | |
| Terry, R. J., 1917. The primordial cranium of the cat.
| |
| Jour. Morph., vol. 29.
| |
| | |
| Valsalva, A. M., 1707. De aure humana tractatus, etc.
| |
| Imolensi, Trajecti ad Rhenum. (Quoted from
| |
| Breschet, 1836.)
| |
| | |
| Vieussens, R., 1714. Traite nouveau de la structure de
| |
| I'oreille. Toulouse. (Quoted from Breschet, 18'6.)
| |
| | |
| Weber-Liel, 1879. Der Aquteductus cochlese des Menschen. Monatschr. f. Ohrenh., xiii, 33-39.
| |
| | |
| Weber, F. E., 1869. Ueber den Zasammenhaug des
| |
| Arachnoidalraumes mit dem LabjTiiith. Monatschr. f. Ohrenh., in, 10.5-107.
| |
| | |
| Weed, L. H., 1917. The development of the cerebrospinal spaces in pig and in man. Contributions to
| |
| Embrj'olog}', vol. 5, No. 14. Carnegie Inst. Wash.
| |
| Pub. No. 225.
| |
| | |
| | |
| | |
| EXPLANATION OF PLATES.
| |
| Plate 1.
| |
| | |
| The figures on Plates I aiui II represent a series of photographs of the ear region inhuman embryos varying from 4 mm.
| |
| to i;{0 mm. long. The photographs were taken at a magnification of 1()0 diameters and as far as possible
| |
| at similar positions, so that a eomparison of them would indicate the actual increase in size and the relative
| |
| amount and form of the individual tissue-masses. In the reproduction tliey were reduced to about 90
| |
| diameters. The different figures include the principal stages in the development of the cartilaginous
| |
| capsule of the car and show the gross features of the histogenesis of the periotic reticulum. Figures 5
| |
| to 7 cover the period during which the mesenchyme becomes condensed around the otic vesicle. Figures
| |
| 8 to 10 show the otic capsule in its precartilage stage and the manner in which the precartilage becomes
| |
| difTerentiated into relatively i)ermanent and temporary zones. The latter encircle the epithelial ducts and
| |
| correspond to the future cartilaginous canals. In figures 1 1 to 13 the main capsular mass has become true
| |
| cartilage, whereius the temporary zone of precartilage surrounding the canal is on the point of dedifferentiating into periotic reticulum. A focal area of vascularized reticulum is already established at the inner
| |
| margin of the epitheUal duct.
| |
| | |
| Fig. 5. Frontal section through the region of the ear in a human embryo 4 mm. long (Carnegie Collection, No. 588,
| |
| slide 6, row 6, section 6). The section is 1.5^ thick and is enlarged 90 diameters. It shows part of the
| |
| brain-wall and t he ot ic vesicle with the surrounding mesenchyme. The nuclei of the latter are more numerous
| |
| in the neighborhood of the vesicle, indicating the beginning of the capsular condensation.
| |
| | |
| Fig. 6. Horizontal section through the region of the ear in a human embryo 9 mm. long (Carnegie Collection, No. 721,
| |
| slide ,"), row 2, section 1). The section is l.'i/i thick and is enlarged 90 diameters. It shows a distinct condensation of the mesenchyme around the otic vesicle, particularly on its lateral surface (above) where it
| |
| extends frotn the surface of the vesicle to about half the distance from the vesicle to the ectoderm.
| |
| | |
| Fig. 7. Frontal section through the labyrinth in a human embryo 11 mm. long (Carnegie Collection, No. 353, shde 16,
| |
| row 3, section 4). The section is 10ft thick and is enlarged 90 diameters. It shows the vestibular part of
| |
| the labyrinth with the appendage opening out of it and passes transversely through the pouches whose
| |
| margins are to form the superior and lateral semicircular ducts. There is now a very complete capsule of
| |
| condensed mesenchyme surrounding every part of the labyrinth, with the exception of the appendage and the
| |
| regions of the interna! auditory meatus and the fenestra cochle«.
| |
| | |
| Fig. 8. Horizontal sec'tioii through the otic capsule in a human embryo 15 mm. long (Carnegie Collection, No. 719,
| |
| slide 3, row 2, section 3). The .section is 40^ thick and is enlarged 90 diameters. It shows a portion of the
| |
| utricle below and the superior semicircular duct above. Surrounding these is a definite capsule of precartilage
| |
| tissue.
| |
| | |
| Fig. 9. Sagittal section through the otic capsule in a human embryo 18 mm. long (Carnegie Collection, No. 144, slide
| |
| 4, row 1, section 3). The section is 40fi thick and is enlarged 90 diameters. Above is the posterior semicircular duct, and just below the center is the lateral semicircular duct. The otic capsule is now differentiated
| |
| into relatively permanent are:is of prccarlilago and other are;is that are more temporary. The latter surround the epithelial ducts and indicate the future cartilaginous canals.
| |
| | |
| Fig. 10. Frontal .section through the otic capsule in a human embryo 27 mm. crown-rump length (Carnegie Collection,
| |
| No. 756a, slide 47, section 2). The section is .")()/i thi<-k and is enlarged 90 diameters. It passes transversely | |
| through the lateral .semicircular canal. The epithelial duct is surrounded by a zone of temporary precartilage
| |
| corresponding to the future cartilaginous canal. Just median to the duct (below it in the photograph) is a
| |
| group of nuclei that forms the focus of the future fp-owth of reticulum.
| |
| | |
| Fio. 1 1 . Section through the lateral semicircular canal in a human fetus 30 mm. crown-rump (Carnegie Collection, No.
| |
| 86, slide 46, section 2). The section is HOit thick and is enlargetl 90 diameters. The main capsular mass is
| |
| now differentiated into true cartilage. The zoik- of temporary precartilage is beginning to recede from the
| |
| epithelial <luct, leaving a reticular area in the interval, which is more pronounced on the median side of the
| |
| duct (below it in the photograph).
| |
| | |
| Fig. 12. .Section through the lateral semicircular canal in a human fetus 37 mm. crown-rump length (Carnegie Collection, No. 972, slide 20, section 1). The section is 50m thick and is enlarged 90 diameters. The nuclei of
| |
| the zone of temporary precartilage form a dark field that corresponds to the future cartiliiginous canal.
| |
| Along the inner margin of this zone are seen large blood-vessels that belong to the periotic reticulum.
| |
| | |
| Fig. 13. Section through the lateral semicircular canal in a human fetus 35 mm. crown-rump length (Carnegie Collection, No. 199, .slide .58, section 2). The section is 50;u thick and is enlarged 90 diameters. It is stained
| |
| deeply with hematoxylin, showing the matrix of the cartilage but not the zone of precartilage that is to become
| |
| the cartilaginous canal.
| |
| | |
| Plate 2.
| |
| | |
| The figures on Plate II are in continuation of those on Plate I and .show the final establishment of the periotic reticular
| |
| tissue. They also show, on being comp.arerl with younger stages, the manner in which the cartilage becomes
| |
| excavated in order lo yield room for the enlarging duct and also to allow for its changing position. The
| |
| excavation is brought about by the dedilTerentiation of cartilage into reticuku- tissue. Throughout this
| |
| period the margin of the cartilaginous canal continues in an unstable condition and is gradually either
| |
| | |
| 52
| |
| | |
| | |
| | |
| EXPLANATION OF PLATES. 53
| |
| | |
| receding or advancing, through the processes of dedifferentiation, into precartilage or differentiation from
| |
| precartilage respectively. The periotic reticulum in its later stages develops fibrous membranes at its inner
| |
| and outer borders. The one at the inner border forms the membrana propria for the epithelial duct, and the
| |
| one at the outer border becomes the perichondrium.
| |
| | |
| Fig. 14. Section through the lateral semicircular canal in a human fetu.s 43 mm. crown-rump length (Carnegie Collection, No. 886, slide 42, section 3). The section is 100m thick and is enlarged 90 diameters. The zone of
| |
| precartilage is expaniling around its peripheral margin by dedifferentiation of the surrounding cartilage and
| |
| on its central margin the precartilage is giving way before the advancing reticulum. A crescentic area of
| |
| periotic reticulum is established on the median side (to the left) of the epithelial duct, about 8 mm. deep in
| |
| the photograph.
| |
| | |
| Fig, 1.5. Section through the lateral semicircular canal in a human fetus 46 mm. crown-rump length (Carnegie Collection, No. 9.5, slide 72, section 1). The section i.s 100m thick and is enlarged 90 diameters. The original area
| |
| of precartilage is now all dedifferentiated into reticulum, and a new area of precartilage has formed outside
| |
| of this at the expense of the smioundiiig cartilage. The new area of precartilage is about OS cm. deep in the
| |
| photograph. Everj-thing between this and the epithelium is reticulum, the peripheral part of which is not
| |
| yet completely vascularized.
| |
| | |
| Fig. 16. Section thiough the posterior semicircular canal in a human fetus 50 mm. crown-rump length (Carnegie
| |
| Collection, No. 184, shde 23). The .section is 50^ thick and is enlarged 90 diameters. The dedifferentiation
| |
| of precartilage into reticulum is nearly complete, there being left ordy a narrow hne of it along the margin of
| |
| the cartilage. The vascularization of the reticulum is not yet completed. The small diameter and the thick
| |
| wall of the epithelial duct in this figure and in figure 15 result from contraction. If they were distended in
| |
| the process of fixation they would doubtless be as large as those in figures 14 and 17.
| |
| | |
| Fig. 17. Section through the posterior semicircular canal in a human fetus 52 mm. crown-rump length (Carnegie
| |
| Collection, No. 96, shde 12, section 2). The section is 100m thick and is enlarged 90 diameters. It differs
| |
| from figui'e 16 in having a more mature periotic reticulum.
| |
| | |
| Fig. is. Section through the posterior semicircular canal in a human fetus, So mm. crown-rump length (Carnegie
| |
| Collection, No. 1400-30, slide 43, section 2). The section is 100m thick and is enlarged 90 diameters. At
| |
| the inner margin of the reticulum can now be seen the membrana propria .supporting the semicircular duct
| |
| and at the outer margin is the thick peri.'hondrium, between which and the cartilage there is a narrow open
| |
| space that is better seen on the left part of the photograph. The sharp dark line along the margin of the
| |
| cartilage on the right is an appearance due to the excavation of cartilage at that point. It consists of an
| |
| intermediate zone in which the cartilage is being dedifferentiated into precartilage and that in turn into
| |
| reticular tissue.
| |
| | |
| Fig. 19. Section through the superior semicircular canal in a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018, slide 30, section 1). The section is 50m thick and is enlarged 90 diameters. It shows a
| |
| rather mature perichondrium closely attached to the cartilage, separated from it, however, by a narrow
| |
| intermediate zone that is not seen in the photograph. ThLs zone is connected with the further enlargement
| |
| of the cartilaginous canal, the growth of which is not yet completed. In the outer part of the canal the
| |
| perichondrium fuses with the membrana propria of the semicircular duct. The periotic reticulum is beginning to break up in the formation of larger spaces, which it does by the retraction of its trabecute, thereby
| |
| allowing adjacent spaces to coalesce. The blood-vessels in this specimen were injected with India ink.
| |
| | |
| Plate 3.
| |
| | |
| The figures on Plate III show the histological appearance of the periotic tissue-spaces and the manner in which they
| |
| are formed from the periotic reticulum. This is accomplished by the disappearance of the trabeculae and the
| |
| consequent repeated coalescence of adjoining spaces.
| |
| | |
| Fig. 20. Section through the second turn of the cochlea in a human fetus 130 mm. crown-rump length (Carnegie
| |
| Collection, No. 1018, slide 32, section 2), enlarged 57 diameters. This section shows the topograph}' of the
| |
| cochlear duct and the general character of the periotic spaces that are developing along its inner margins.
| |
| Details of this same section as seen under higher magnification are shown in figures 22 and 24.
| |
| | |
| Fig. 22. Detail of the section shown in figure 20, enlarged 278 diameters. This figure shows the part of the coclilear
| |
| duct that is to form the organ of Corti with the adjacent tissue that becomes incorporated in the basilar membrane. Below is the periotic reticulum, whose spaces are in the process of enlarging. By repeated coalescence these spaces finally unite with the large space which constitutes the scala tympani. This figure shows
| |
| the histological appearance of the reticulum where the formation of ti.ssue-spaces is in active operation.
| |
| | |
| Fig. 24. Detail of the section shown in figure 20, enlarged 300 diameters. It shows the character of the margin of the
| |
| scala vestibuli in a fairly mature condition. The scala vestibuli is inclosed by a membrane consisting of the
| |
| cells that had previously constituted the reticulum occupying this area and which have been modified in form
| |
| in adaptation to the formation of this large tissue-.space, closing it off from the surrounding tissue.
| |
| | |
| Fig. 21 . Section through the vestibular portion of the labyrinth in a human fetus 52 mm. crown-iump length (Carnegie
| |
| Collection, No. 448, slide 154, section 2), enlarged 31 diameters. This section shows the general character
| |
| of the periotic spaces and their relation to the different parts of the membranous labyrinth and the surrounding cartilaginous capsule. The first space to develop and the largest shown in this figure is the vestibular
| |
| cistern, situated between the utricle and the cartilaginous stapes. The smaller spaces, belowthe cistern
| |
| and extending downward along the cochlear duct, represent the scala vestibuli in an early form. The
| |
| arteries in this specimen were injected with Imlia ink and are shown in black.
| |
| | |
| | |
| | |
| 54 EXPLANATION OF PLATES.
| |
| | |
| Fir,. iV Section tlirough the superior semicircular canal in a human fetus 130 mm. crown-rump length (Carnegie
| |
| Collection, \o. 1018, slide 29, section 2), enlarged 90 diameters. The periotic reticulum Ls undergoing the
| |
| alterations characteristic of the earlj' st^es of the formation of tissue-spaces. Along the margins of the
| |
| cartilage the reticular tissue is condensed and (^oiLstitutes the fibrous pcrichoiulrium. .\round the epithelial
| |
| canal there is developed a layer of supporting tissue h hicli forms the iiieinbrana propria. This layer fuses
| |
| with the perichondrium along the jx-ripheral margin of thi- ciiiial :in<l thereby constitutes a ligament that
| |
| attaches each membranous duct throughout its whole length to the cartilaginous .space in which it is suspended.
| |
| | |
| Fin. 2.'i. Section through the apex of the cochlea of a human fetus 130 mm. crown-rump length (Carnegie Collection,
| |
| No. 1018, slide 32, section 2), enlarged 57 diameters. This section shows the tip of the cochlear duct and
| |
| the character of the communication that develops between the two scate forming the helicotrenia. It
| |
| will be seen that the margins of the periotic spaces are not so mature here as in the proximal parts of the
| |
| cochlea of the same fetua, on comparing tliis figure with figure 20.
| |
| | |
| Plate 4.
| |
| | |
| The figures shown on this plate represent a series of median and lateral views of wax-plate reconstructions of the
| |
| membranous labyrinth and the surrounding periotic tissue-spaces. They illustrate under the same scale of
| |
| enlargement three typical stages in the development of these spaces. Abbreviations: C. s. 1., ductus
| |
| semicircuiaris lateralis; C. s. p., ductus semicircularis posterior; C. s. s., ductus semicircularis superior;
| |
| Duct, coch., ductus cochlearis; Impressio rotund., area opposite the fenestra cochleae; Impressio staped.,
| |
| area in contact with base of stapes; Saccus endol., saccus endolymphaticus; Scala tymp., scala tynipani;
| |
| Scala vestib., scala vestibuH.
| |
| | |
| Fio. 26. Lateral view of a model reconstructed from a human fetus 50 mm. crown-rump length (Carnegie Collection,
| |
| No. 84). The cistern and the scala vestibuli are shown in green and the scala tympani is shown in orange.
| |
| The scala vestibuU is in the first stage of its development and consists of a row of large reticular spaces which
| |
| extend from the ventral margin of the cistern downward along the apical surface of the cochlear duct. The
| |
| scala tympani is more advanced and shows more complete coalescence of its constituent spaces. Enlart;eii
| |
| 1 1 .4 diameters.
| |
| | |
| Fin. 27. Median view of the same model .shown in figure 26. This view shows the tojjography of the scala tympani.
| |
| Its large proximal end lies opposite the fenestra cochleje (rotunda) and corresponds to the focus at which its
| |
| development originates. Distally it tapers off rapidly where the spaces are smaller and their coalescence less
| |
| complete. Enlarged 11.4 diameters.
| |
| | |
| Fi(i. 28. Lateral view of wax-plate reconstruction of the left membranous labyrinth and the periotic spaces in a human
| |
| fetus 85 mm. crown-rump length (Carnegie Collection, No. 1400-30), enlarged 11.4 diameters. The cistern
| |
| and the connecting scala vestibuli are shown in green. Although the greater jiart of the cistern abuts against
| |
| the stapes, it will be noted that it is also begiiming to spread over the liorsal surface of the utricle and along
| |
| the inner border of the lateral semicircular duct. The scala vestibuh communicates freely with the cistern
| |
| and extends downward alotig the apical surface of the cochlear duct, throughout nearly two turns, showing
| |
| the characteristic sacculated appearance near its tip, w here the coalescence of the spaces is less complete.
| |
| | |
| Fi<;. 29. Median view of same model shown in figure 28, enlarged 11.4 diameters. The scala tympani is shown in
| |
| orange. The oval indentation in its proximal end corresponds to the fenestra cochlea (rotunda). This
| |
| space extends along the cochlear duct about the same distance as the scala vestibuli, but the two do not comMUinicate yet at any place. The peripheral border of the scala tympani is characterized by sacculations
| |
| corresponding to spaces that are coalescing with the main s|)a(c. The grow th of the scala is due to a coalescence of new spaces along its peripheral border rather than along its cential border.
| |
| | |
| Fio. 30. Lateral view of a wax-plate reconstruction of the li'ft membnmoiis labyrinth and the periotic spaces in a
| |
| human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018), enlarged 11.4 diameters. The
| |
| cistern and scala vestibuli are shown in green and the scala tympani is shown in orange, as in the previous
| |
| figures. The cartilaginous stapes was removed from this model and the oval impression that it makes on the
| |
| cistern can be plaiidy seen. The cistern has spread over the top of the utricle and part way along the lateral
| |
| semicircular duct. The scala vestibuli extends to the ti|) of the cochlear duct, where it communicates with
| |
| the McaLi tympani, thas forming the helicotrema.
| |
| | |
| Fki. 31 . Mcnlian view of .same model shown in figure 30, enlarginl 1 1.4 diameters. The oval impression on the proximal i-nd of the scala tympani corresponds to the fenestra cochle;c (rotunda). .\s yet there is no conmiunication at this point between the scala tympani and subarachnoid spaces, such :is is found in the adult and
| |
| known as the aqua-ductus cochlete. The spaces making up the <ist<'rn cover almost the whole of th<' utricle
| |
| and saccule except the places at whicli t he nervi-s enter and a small part of the medial surface near the attachment of tlie appendage.
| |